Residual Solvents Testing of Packaging Materials per ASTM F1884-04 with the HT3 Automated Headspace Sampler
Printing inks used on packing materials of consumer products contain colorants and pigments, binder systems, solvents, and additives. ASTM F 1884-04 (1), Standard Test Methods for Determining Residual Solvents in Packaging Materials determines residual solvents in packaging materials from printing processes utilizing solvent-based printing inks.
Printing inks used on packing materials of consumer products contain colorants and pigments, binder systems, solvents, and additives. ASTM F 1884-04 (1), Standard Test Methods for Determining Residual Solvents in Packaging Materials determines residual solvents in packaging materials from printing processes utilizing solvent-based printing inks.
Test method A recommends an autosampler to perform the headspace-gas chromatography examination of residual solvents in packaging materials. The Teledyne Tekmar HT3 automated headspace sampler will be used to meet the rigorous demands of ASTM F 1884-04. This method prepares a standard curve in the package sample matrix.
Standard Preparation
A 20 μL/L 4-heptanone solution was used as an internal standard. A five-point calibration curve was prepared by diluting a commercially available residual solvent standard mix with the internal standard solution. A calibration curve was prepared by adding 2 mL of the standard or internal standard solutions into 22 mL headspace vials.
The samples were automatically injected onto a GC/FID system by the Tekmar HT3 automated headspace sampler.
Results
The relative standard deviation for the average of the response factors (%RSD) and correlation coefficients (r2) were calculated by the internal standard method for the five-point calibration curve. An example of this calibration data (labeled Peak #9) is presented in Figure 1. Figure 2 shows a comparison of a calibration standard to a film blank standard.

Figure 1: Calibration data for a residual solvent compound analyzed by ASTM F1884-04.
Conclusions
The Teledyne Tekmar HT3 automated headspace sampler provides excellent linearity for residual solvent standards used in the analysis of residual solvents in packaging material as defined by ASTM method F1884-04, Standard Test Methods for Determining Residual Solvents in Packaging Materials.
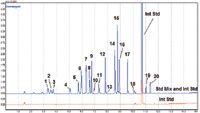
Figure 2: Overlay chromatograms of a blank (bottom) and a high-level standard (top).
Additional data for the HT3 and the new Versa automated headspace sampler using ASTM Method F1884-04 will be presented at the 2012 Eastern Analytical Symposium and Exhibition.
References
(1) ASTM Method F1884-04, Standard Test Methods for Determining Residual Solvents in Packaging Materials, ASTM International, West Conshohocken, PA, United States.
Teledyne Tekmar
4736 Socialville Foster Rd., Mason, OH 45040
tel. (800) 874-2004
Website: www.teledynetekmar.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














