Recycle Reversed-Phase Liquid Chromatography to Achieve Separations Based on One H/D Substitution on Aromatic Hydrocarbons
Special Issues
Ultrahigh-efficiency separations based on the presence of one deuterium in benzene, toluene, and naphthalene were achieved by recycle chromatography using C18 silica columns. Larger isotopic separation factors, α(H/D), were observed in methanol–water than in acetonitrile–water, when the mobile phases provided similar retention factors (k), or similar methylene selectivity, α(CH2). Isotopic resolutions between nondeuterated and perdeuterated aromatic hydrocarbons at long separation times were estimated by using the plate counts obtainable by recycle operation as a function of a cycle time, along with the retention factors and the separation factors experimentally observed.
Photo Credit: chris/stock.adobe.com

Ultrahigh-efficiency separations based on the presence of one deuterium in benzene, toluene, and naphthalene were achieved by recycle chromatography using C18 silica columns. Larger isotopic separation factors, α(H/D), were observed in methanol–water than in acetonitrile–water, when the mobile phases provided similar retention factors (k), or similar methylene selectivity, α(CH2). Isotopic resolutions between nondeuterated and perdeuterated aromatic hydrocarbons at long separation times were estimated by using the plate counts obtainable by recycle operation as a function of a cycle time, along with the retention factors and the separation factors experimentally observed. Methanol–acetonitrile–water ternary mobile phases were predicted to provide the greatest resolution per unit time, and actually enabled separation by the difference of one H/D substitution on the aromatic hydrocarbons with α(H/D) = 1.008 or less, and also the differentiation of the isomers of monodeuterated toluene with α(toluene-4-d/toluene-α-d) = 1.0016. The discrimination mechanism of H/D isotopic species is discussed based on the dispersion interactions of a CH/CD group of the solute with the stationary phase as well as the mobile phase.
The separation of isotopic compounds has often been performed to demonstrate the high efficiency of a chromatographic system, and also to satisfy scientific curiosity about how small the difference can be for two kinds of solutes to be separated by liquid chromatography (LC), the presence of only one deuterium (D) or its position in a molecule.
The separation of hydrogen isotopic compounds illustrated the advances of C18 silica columns (1,2,3,4,5,6,7). Other examples include separations based on isotopic chirality created by the presence of deuterium in place of hydrogen (8,9). The differentiation of H/D-isotopically chiral molecules indicates the presence of specific interactions involving the isotopic substituents on the chiral centre, as suggested by mechanistic studies on CH/CD discrimination by reversedâphase high performance liquid chromatography (HPLC) (10,11,12,13).
In order to generate large plate counts, a long column or a series of several columns (6,14,15) and/or recycle chromatography (3,4,5,7,8,9,14,15) have been used for isotopic separations. Solutes can be recycled through the column by simply connecting the detector outlet tube to the pump inlet (Figure 1[a]) (8,9) or by alternate column recycle chromatography (ACRC, Figure 1[b]) (3,4,5,7,14,15). The extracolumn volume should be minimized in both cases, and preferably a large column should be used in Figure 1(a). In the case of ACRC, the flow switching valve should be operated when the solute bands are located in the middle of a column so the whole bands can be held in one of the two columns as long as possible.
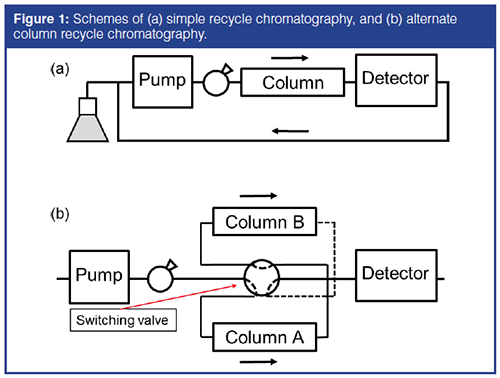
In most cases, acetonitrile–water mixtures were preferred to methanol–water as a mobile phase in reversedâphase mode because the lower viscosity enables the use of a long column and high flow rate, which can generate large plate counts. This approach can be justified when the isotopic separation is used to demonstrate the high efficiency of a column or an instrument. In an attempt to achieve the ultimate discrimination of the isotopic compounds, however, the separation conditions can be further optimized to produce high selectivity as well as high efficiency, as practiced in routine HPLC applications.
Both stationary phase and mobile phase participate in the discrimination between nondeuterated and deuterated compounds in reversed-phase HPLC (3,11,12,13). While silica-based stationary phases containing heavy atoms (pentabromobenzyloxypropyl, PBB), or poly-aromatic structures (pyrenylethyl, PYE, and fullerene-bonded, C70), resulted in an increased retention and high H/D isotopic selectivity (11,13), C18 stationary phases have been used for the separation of one or threeâDâsubstituted benzenes (3,4,5,6,7), owing to the high efficiency and high selectivity for CH/CD in aromatic compounds.
Methanol–water mixtures were reported to provide larger H/D separation factors for the isotopologues of benzene and toluene than acetonitrile–water (3,11,12,13). This article reports on the optimization of the mobile phase composition, including a methanol–acetonitrile–water ternary mobile phase. The application of the optimized conditions to separate benzene and toluene isotopologues with the difference of one deuterium substitution with a C18 column system (150 mm × 4 = 60 cm total length, 6-mm internal diamter [i.d.]) will be described. The results will be compared with those reported earlier, including the recent paper (7), while mechanistic interpretations for CH/CD discrimination will be provided in relation to the mobile phase selection.
Experimental
Simple recycle chromatography (Figure 1[a]) was performed with conventional HPLC, LC-9A (Shimadzu), equipped with a SPDâ6A UV detector (8-μL cell, operated at 254 nm) and a valve-loop injector. The 20 μL sample loop of the injector was bypassed during the recycle operation. The ultrahigh-pressure liquid chromatography (UHPLC) instrument, Nexera (Shimadzu), equipped with a pump LC-30AD and a UV detector SPD-20A, was used for the singleâcolumn experiment with a 15 cm × 3 mm, 5-μm Cosmosil 5-C18âII column (Nacalai Tesque). A series of four 15 cm × 6 mm, 5-μm Cosmosil 5-C18-II columns (Nacalai Tesque) were used for recycle chromatography (the column dead volume, Vm, 10.6 mL). Chromatographic measurement was performed at 30 ºC. Deuterated compounds were available from C/D/N Isotopes Inc. or SigmaâAldrich. Other chemicals were obtained from Nacalai Tesque.
Results and Discussion
Plate Counts, Retention Factors, and Separation Factors in Relation to the Mobile Phase Composition: Maximum plate counts, 11080 and 9900, were observed for benzene in 45:55 acetonitrile–water at chromatographic linear velocity, u = 2.5 mm/s (0.6 mL/min), and in 45:55 methanol–water at 1.5 mm/s (0.4 mL/min), respectively, with the 3-mm i.d. column. (Up to 25% larger plate counts were observed with the 6-mm i.d. columns because of the smaller contribution of the extracolumn dispersion.)
A ternary mobile phase, 25:20:55 methanol–acetonitrile–water, provided the van Deemter plot in between those obtained for the binary mobile phases, with maximum 10,840 theoretical plates at 2 mm/s (0.5 mL/min). The effect of the plate counts on resolution (RS, equation 1) is not large compared to the effects of the retention factor, k, and the separation factor, α, in the linear-velocity range 1.5–2 mm/s used for recycle chromatography:
RS = (1/4) (√N) (α-1) [k/(k+1)] [1]
Figure 2(a) shows the plot of separation factors between toluene and benzene (the right y-axis), α(CH2) = (ktoluene/kbenzene), and α(H/D) values (the left y-axis) observed for nondeuterated and perdeuterated benzenes against organic solvent content. The α(H/D) and α(CH2) are larger in methanol–water than in acetonitrile–water at the same organic content, and decrease with the increase in organic solvent content. For example, α(CH2) in 30:70 acetonitrile–water is similar to that in 55:45 methanol–water, and the retention factor of benzene-d6 in 35:65 acetonitrile–water is similar to that observed in 45:55 methanol–water (using uracil as a t0 marker).
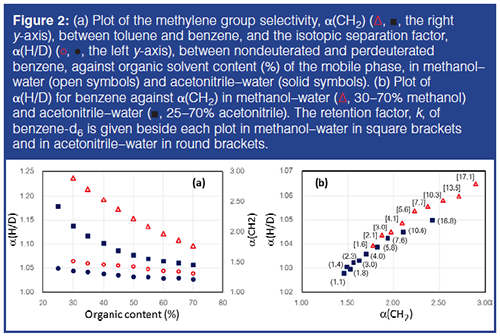
Figure 2(b) shows the plot of α(H/D) against α(CH2) with the retention factor of benzene-d6 attached. The α(H/D) in methanol–water are clearly larger than those in acetonitrile–water when α(H/D) are compared at similar α(CH2) values, in spite of the smaller retention factors in methanol–water, as reported by Tchapla et al. (12). It is interesting to note that α(H/D) values are different for the mobile phases producing the same α(CH2) value, indicating that CH and CD are differentiated by the mechanism that is different from typical hydrophobic interactions causing certain increase in retention by the addition of a methylene group to the solute structure, and the latter mechanism results in larger α(H/D) in methanol–water than in acetonitrile–water (Note that the plots merge in water in the absence of the organic solvent). This subject will be discussed later. The shorter retention allowing a faster recycle operation and larger isotopic separation factors should lead to easier H/D isotopic separations in methanol–water than in acetonitrile–water.
The plate counts obtainable in recycle chromatography for a solute can be considered in terms of the effective column length, or the actual column length multiplied by the number of cycles. The number of theoretical plates obtainable for each 1 h separation time, N(RC)/h, can be calculated from the observed plate counts, N(obs), by equation 2, where tR is the total separation time in minutes:
N(RC)/h = N (obs)/(tR/60) [2]
N(RC)/h values plotted against tR in Figure 3(a) indicate that the first few cycles of recycle operation are accompanied by a considerable decrease in plate counts for each hour as a result of the small band width in an early stage of recycle chromatography, particularly for the conditions that resulted in small retention factors. This is presumably a result of the contribution of extracolumn effects, including those of the detector and the pump. After several cycles, the decrease in N(RC)/h settled because of the increase in the band width. The larger the retention factor of a solute (longer time for the first point of the plot), the larger the plate counts that were obtained for each cycle (data not shown), and the smaller the decrease in N(RC)/h that was observed with increasing number of recycles.
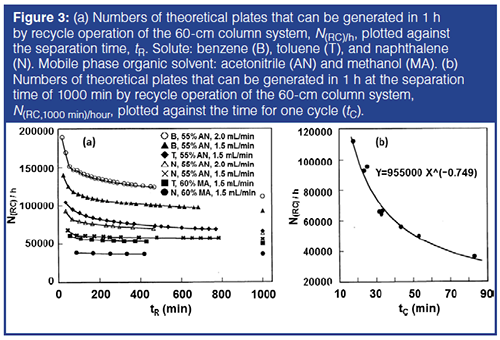
The largest N(RC)/h was observed for the higher flow rate, that is, for the smallest cycle time, tC (time required for one cycle, tC = t0[k + 1]), and for the smallest retention factor, k. Thus, a faster flow rate and shorter retention are advantageous for generating large numbers of theoretical plates, in spite of the smaller number of theoretical plates for each cycle. The plate counts obtainable per unit time (h) at the separation time of tR = 1000 min, N(RC,1000 min)/h, were calculated by the extrapolation of the last part of each curve in Figure 3(a). The observed column efficiency at 1000 min was 33,000–48,000 theoretical plates for each cycle for different cycle times. The plots of the obtained N(RC,1000 min)/h against tC in Figure 3(b) were found to be approximated by equation 3 with r2 = 0.86. This is actually a rough estimate because the plate counts were obtained at different flow rates in a narrow range, and for different solutes and mobile phases:
N(RC,1000 min)/h = 955000 tC-0.749 [3]
The N(RC,1000 min)/h values described by equation 3 in Figure 3(b) were used for estimating the resolution of two isotopologues at long separation times in combination with the retention factors and the separation factors experimentally observed. The N(RC,1000 min)/h values calculated by equation 3 give a conservative estimate of the resolution for a shorter time of recycle operation than 1000 min, because the N(RC)/h values for a shorter separation time are larger than N(RC,1000 min)/h, as shown in Figure 3(a).
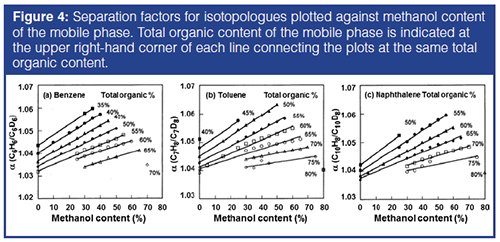
Figure 4(a) shows the plots of the isotopic separation factor, α(H/D), for benzene–benzene-d6 against the methanol content of the binary or ternary mobile phases observed with a 6-mm i.d. column, while the total organic solvent content is indicated in the upper right-hand corner of the plots in the figure. The plots obtained at the same total organic content are connected with a line. The separation factors, α(H/D), (and the retention factors of benzene, toluene, and naphthalene) monotonously decreased with the increase in acetonitrile content of the mobile phase at a constant total organic content in all cases. It should also be noted that the isotopic separation factors, α(H/D) values, are larger in methanol–water than in acetonitrile–water, producing similar α(CH2), or similar retention factor, k, as shown in Figure 2(b). Similar plots are made for toluene and naphthalene isotopologues. When the acetonitrile content in the ternary mobile phase is 20% or more, the pressure limit of 30 MPa allows the flow rate of 2 mL/min through the recycle system. With such a mobile phase composition, the separation factors are closer to those in methanol–water, and the sample band may be recycled at a high flow rate.
The resolution between a nondeuterated and perdeuterated solute pair obtainable in 1 h, RS/h, at a long recycle separation time, 1000 min, can be calculated by equation 4 as a function of a retention factor, k, and a separation factor, α(H/D), with the plate counts, N(RC,1000)/h, where N in equation 1 is given by equation 3:
RS/h = (1/4) [√(955000 [t0(k+1)]–0.749)] (α-1) [k/(k+1)] [4]
RS/h values for benzene and benzene-d6 for the mobile phases examined in Figure 4 were calculated from the N(RC,1000)/h values in Figure 3(b) with the cycle time, tC, and the separation factors shown in Figure 4. The optimum mobile phases are predicted to be 25:20:55 or 30:15:55 methanol–acetonitrile–water for the separation of benzene and benzene-d6, and 40:20:40 methanol–acetonitrile–water for toluene and toluene-d8. Similarly, 45:20:35 methanol–acetonitrile–water was predicted to be optimum for the separation between naphthalene and naphthalene–d8. The RS/h values calculated for nondeuterated and perdeuterated solute pairs were 2.54, 2.94, and 3.34 for benzene, toluene, and naphthalene, respectively, in the optimum ternary mobile phases.
Separation of Benzene and Deuterated Benzenes Based on One H/D Substitution: Figure 5 shows the separation of isotopic benzenes, benzene-d6, -d5, -1,3,5-d3, -d, and benzene, by recycle chromatography with the optimal mobile phase composition, 25:20:55 methanol–acetonitrile–water. The chromatogram in Figure 5(b) shows plate counts of approximately 500,000, resulting in the separation of the benzene isotopologues with a resolution greater than 1.0 for the difference of one H/D substitution in approximately 460 min. The resolution, RS/h = 2.54, obtainable in 1 h under the present conditions estimated based on equation 4, indicates that RS = 4.0 for benzene–benzene-d6, or RS = 2.0 based on three deuterium substitutions, can be obtained in approximately 160 min with about 170,000 theoretical plates and α(H/D) = 1.023. The results will be compared with those from other studies.
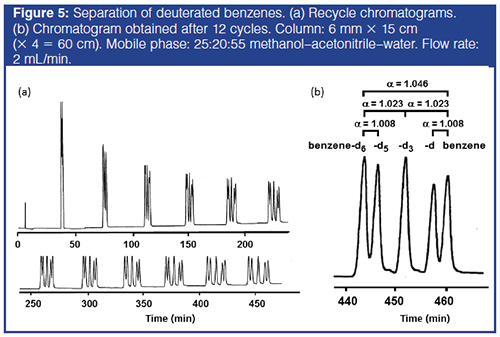
Similar benzene–benzene-d separation was achieved by van der Wal in approximately 700 min with 55 cycles by ACRC with two 15-cm columns in 20:80 acetonitrile–water generating N = 200,000 and α(H/D) = 1.049 (4). He intentionally used a less selective mobile phase to illustrate the efficiency of the instrument and the column. The increases in k and α(H/D) were observed during ACRC operation, indicating the effect of pressure on retention in the presence of steep pressure gradient, 38 MPa/30âcm column length. Greater pressure effects were observed for nitrogen or oxygen isotopic separations in ionization control mode (14,15). Takeuchi and co-workers reported RS of approximately 2.0 for benzene–benzene-1,3,5-d3–benzene-d6 with N = 230000 in about 600 min in 30:70 acetonitrile–water at 0.8 MPa by ACRC using monolithic silica capillary C18 columns (0.1 mm i.d., 45.5 and 44.0 cm) (5). In these examples, low acetonitrile content mobile phases were employed to obtain a large α(H/D).
Recently, Gritti and co-workers reported extremely fast separation of benzene–benzene-1,3,5-d3–benzene-d6 with RS of 2.0, α(H/D) = 1.023, and k = 2.0 by ACRC using two columns packed with core–shell silica C18 particles (15 cm × 3 mm, 2.7-μm) in 83 min in 22 cycles to generate N = 275,000, at a flow rate of 0.4 mL/min under 5300 psi (approximately 37 MPa/30 cm) (7). The reported separation factor, α(H/D) = 1.023, for the difference of three deuterium atoms on benzene, or α(H/D) = 1.046 for benzene–benzene-d6 in 55:45 acetonitrile–water, was much larger than that reported by Tchapla and co-workers (12) and was also observed in the present study. Although the effect of pressure on retention and separation factor was not reported, the pressure gradient was similar to that reported by van der Wal (4). The polymeric C18 phase employed could show larger α(H/D) than the monomeric C18, especially under high pressure (16), making the system sensitive to the impact of pressure.
The examples discussed in this section indicate the importance of optimization of mobile phase, and the importance of understanding the effect of pressure on solute retention for ACRC using short, high efficiency columns under steep pressure gradient. In the present system, the pressure effect was avoided with small pressure gradient 30 MPa/60 cm and by employing 5 μm, monomeric C18 silica particles. The use of a polymeric C18 phase in combination with an optimized mobile phase may further facilitate the H/D isotopic separations.
Separations of Isotopologues Based on One H/D Substitution with Toluene and Naphthalene: Figure 6(a) shows separations of toluene-α-d and toluene-4-d from toluene in 40:20:40 methanol–acetonitrile–water. The results indicate a very slight difference in retention factor between tolueneâα-d and toluene-4-d. As shown in Figure 6(b), the two monodeuterated toluene isomers were differentiated based on a separation factor of 1.0016 by the long recycle operation generating 3,500,000–4,000,000 theoretical plates in about three days. A slightly larger H/D isotope effect for aromatic CH/CD than aliphatic CH/CD was reported previously (11,12). Figure 6(c) shows the separation between naphthalene and naphthalene-2-d in 45:20:35 methanol–acetonitrile–water. The results suggest that such isotopic isomers can be used to demonstrate the high efficiency of modern chromatographic systems and columns. The use of optimized conditions is suggested.
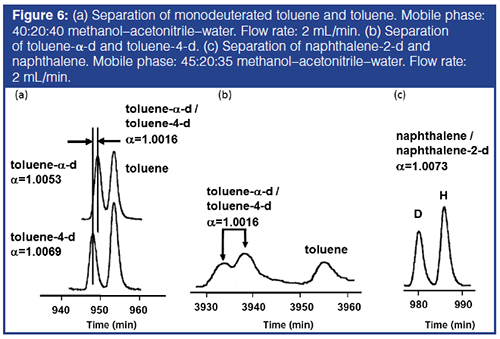
In the case of monodeuterobenzoic acid, the H/D isotope effect was found to be in the order, p- > m- > o-deuterobenzoic acid (17). The isotope effect observed with tolueneâα-d was smaller than that for toluene-4-d. This can be explained by the steric effect of the substituent, or a neighbouring group, partially shielding the CH/CD bond from intermolecular interactions.
Mechanistic Interpretations of H/D Isotopic Selectivity: Polarizability or lipophilicity of a stationary phase is affected by the density of the alkyl groups, as indicated by the larger α(CH2), representing the behaviour of a typical hydrophobic group, with high coverage C18 than low coverage C18 in the same aqueous mobile phase (18). High polarizability stationary phases, such as PBB and PYE, provide larger α(H/D) values for aliphatic CH/CD along with the smaller α(CH2) than C18 (11). These results indicate the contribution of attractive interactions between a solute and the stationary phase to retention, namely the dispersion interactions (instantaneous dipole-induced dipole interactions) that play a major role for H/D isotopic separations because of the greater polarizability of the CH than the CD group (10,11,12).
The greater α(H/D) values in methanol–water than acetonitrile–water at the same α(CH2) values (Figure 2[b]) can be explained by the greater contribution of dispersion interactions in the former. When acetonitrile–water and methanol–water provide similar α(CH2), the free energy changes associated with the transfer of one methylene group (CH2) from the mobile phase to the stationary phase are similar. Acetonitrile is known to be absorbed by the stationary phase more than methanol (19), making the C18 stationary phase less hydrophobic and less polarizable than the C18 phase in methanol–water, even when the two systems provide similar α(CH2) values. In other words, similar α(CH2) can be obtained when an acetonitrile–water mobile phase is more aqueous than methanol–water. The intrinsic polarizability of the stationary phase is more strongly reflected in solute retention in methanol–water than in acetonitrile–water, resulting in the larger H/D isotope effects.
The amount of organic solvent extracted into the stationary phase from the mobile phase may also cause the difference in the phase ratio, influencing the retention factors. Acetonitrile–water provides considerably larger retention factors for a variety of solutes than methanol–water, when the two types of mobile phase provide similar α(CH2) values (20), as observed in this study (Figure 2[b]). Hydrophobic solutes tend to be associated with organic solvent molecules in aqueous mobile phases, which can also contribute to the smaller α(H/D) in a mobile phase containing acetonitrile, which is more polarizable than methanol. This way, the larger isotope effects observed in methanol–water than in acetonitrile–water can be rationalized, and should be considered for H/D isotopic separations.
Conclusions
Separations based on one H/D substitution on aromatic hydrocarbons were achieved by recycle chromatography in the reversedâphase mode, and the discrimination of isomers with a difference in the position of only one deuterium atom was demonstrated using 5-μm C18 silica particles and using ternary mobile phases, methanol–acetonitrile–water, selected to provide the best compromise between isotopic selectivity and column backpressure.
References
- N. Tanaka and E.R. Thornton, J. Am. Chem. Soc.98, 1617–1619 (1976).
- G.P. Cartoni and I. Ferretti, J. Chromatogr. 122, 287–291 (1976).
- S.J. van der Wal, J. Liquid Chromatogr.8, 2003–2016 (1985).
- S.J. van der Wal, Chromatographia22, 81–87 (1986).
- L. Lim, H. Uzu, and T. Takeuchi, J. Sep. Sci.27 1339–1344 (2004).
- K. Miyamoto, T. Hara, H. Kobayashi, H. Morisaka, D. Tokuda, K. Horie, K. Koduki, S. Makino, O. Nunez, C. Yang, T. Kawabe, T. Ikegami, H. Takubo, Y. Ishihama, and N. Tanaka, Anal. Chem.80, 8741–8750 (2008).
- F. Gritti and S. Cormier, J. Chromatogr. A1532, 74–88 (2018). (and additional personal communication)
- K. Kimata, M. Kobayashi, K. Hosoya, T. Araki, and N. Tanaka, J. Am. Chem. Soc.118, 759–762 (1996).
- K. Kimata, K. Hosoya, T. Araki, and N. Tanaka, Anal. Chem.69, 2610–2612 (1997).
- N. Tanaka and E.R. Thornton, J. Am. Chem. Soc. 99, 7300–7307 (1977).
- M. Turowski, N. Yamakawa, J. Meller, K. Kimata, T. Ikegami, K. Hosoya, N. Tanaka, and E.R. Thornton, J. Am. Chem. Soc.125, 13836–13849 (2003).
- A. Valleix, S. Carrat, C. Caussignac, E. Léonce, and A. Tchapla, J. Chromatogr. A1116, 109–126 (2006).
- E. Kanao, T. Kubo, T. Naito, T. Matsumoto, T. Sano, M. Yan, and K. Otsuka, J. Phys. Chem. C122, 15026−15032 (2018).
- N. Tanaka, A. Yamaguchi, K. Hashizume, M. Araki, A. Wada, and K. Kimata, J. High Resolut. Chromatogr. 9, 683–687 (1986).
- N. Tanaka, K. Hosoya, K. Nomura, T. Yoshimura, T. Ohki, R. Yamaoka, K. Kimata, and M. Araki, Nature 341, 727–728 (1989).
- K. Okusa, Y. Iwasaki, I. Kuroda, S. Miwa, M. Ohira, T. Nagai, H. Mizobe, N. Gotoh, T. Ikegami, D.V. McCalley, and N. Tanaka, J. Chromatogr. A1339, 86–95 (2014), supplementary information.
- W.J.S. Lockley, J. Chromatogr. A483, 413–418 (1989).
- K. Kimata, K. Iwaguchi, S. Onishi, K. Jinno, R. Eksteen, K. Hosoya, M. Araki, and N. Tanaka, J. Chromatogr. Sci.27, 721–728 (1989).
- R.M. McCormick and B.L. Karger, Anal. Chem.52, 2249–2257 (1980).
- N. Tanaka, H. Goodell, and B.L. Karger, J. Chromatogr.158, 233–248 (1978).
Kazuhiro Kimata has retired from Nacalai Tesque, Inc.
Tsunehisa Hirose belongs to the research and development division of Nacalai Tesque, Inc.
Eisuke Kanao is a graduate student at the Graduate School of Engineering, Kyoto University, in Kyoto, Japan.
Takuya Kubo is an Associate Professor at the Graduate School of Engineering, Kyoto University.
Koji Otsuka is a Professor at the Graduate School of Engineering, Kyoto University.
Ken Hosoya is a Professor in the Graduate School of Life and Environmental Sciences, Kyoto Prefectural University, in Kyoto, Japan.
Kohei Yoshikawa is a graduate student in the Department of Biotechnology, Graduate School of Engineering, Osaka University, in Osaka, Japan.
Eiichiro Fukusaki is a Professor in the Department of Biotechnology, Graduate School of Engineering, Osaka University.
Nobuo Tanaka is an Invited Professor in the Department of Biotechnology, Graduate School of Engineering, Osaka University.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)






