Vacuum-Jacketed Columns: Maximum Efficiency, Easy Deployment Without Oven, and Improved LC–MS Performance
Special Issues
This article demonstrates how a user-friendly vacuum-jacketed column (VJC) has been designed without the need of a large internal diameter vacuum chamber and low- and high-vacuum pumps.
Photo Credit: chris/stock.adobe.com
This article demonstrates how a user-friendly vacuum-jacketed column (VJC) has been designed without the need of a large internal diameter vacuum chamber and low- and high-vacuum pumps. Efficiency tests show that the VJC cannot be run under strict adiabatic condition because of the small residual heat loss at both ends of the VJC, but 95% of the maximum expected efficiency is achieved. It is also shown that the VJC can be advantageously directly deployed to any optical detector, which avoids the need for extracolumn tubing, and omits the need for a column oven to operate at high temperatures. Finally, this work describes how to improve the coupling between the eluent preheater, the chromatographic column, and the electrospray ionization (ESI) probe for mass spectrometry (MS) detection. Besides a 30% gain in column efficiency under extreme viscous heating conditions (10 Watt/m), the VJC-MS probe eliminates most of the post-column sample dispersion of conventional liquid chromatography (LC)–MS systems. Overall, the experimental peak capacities measured for a 2.1 × 100 mm column packed with sub-2-µm particles and placed in the VJC-MS probe are doubled with respect to standard LC–MS systems.
In a recent article published in a special supplement of LCGC (1), the development of a chromatographic column that can be operated under strict adiabatic conditions (2) was described. The main objective was to maintain the intrinsic resolution power of chromatographic columns when operating them under extreme experimental conditions involving undesirable thermal effects, and leading to inevitable losses in column performance. These applications cover analyses by either ultrahigh-pressure liquid chromatography ([UHPLC], severe eluent heating [3–8]) or by lowâdensity fluid chromatography at high temperatures and low backpressures, such as supercritical fluid chromatography ([SFC], JouleâThomson decompression, severe eluent cooling [9]). Experimental evidence was shown that if a chromatographic column is fully embedded in a large cylindrical chamber (6-cm internal diameter [i.d.] and 25-cm-long) in which a high vacuum (air pressure ~ 10-5 Torr) is applied, and if all surface area (column and vacuum chamber) are wrapped with a thin aluminium foil, then the maximum expected performance of the column was systematically achieved regardless of the intensity of the thermal effects (10–12). The advantage of placing chromatographic columns in a strict adiabatic environment was then established.
However, these “proof-of-concept adiabatic” columns are highly impractical: routine high-throughput LC analyses cannot comply with adopting a heavy (~ 5 kg) stainless steel (SS) vacuum chamber, complex accessories related to vacuum technology (among others, a lowâvacuum oil pump and a highâvacuum turbomolecular pump), extremely long setup times (half a day) to assemble and connect the whole column/vacuum chamber/instrument system, and a one night equilibration time to degas the vacuum chamber (volume ~ 4 litres) down to 10-5 Torr. Efforts are first needed towards reducing the large volume of the vacuum chamber while keeping both the oil and turbomolecular pumps. This was successfully achieved with a 2.0âcm i.d. cylindrical chamber after reduction of the thermal mass of both column endfittings, and sealing the SS chamber with two insulating PEEK side flanges (1). The small chamber (volume ~ 0.05 litre) still provided the column a strict adiabatic environment, heat transfer by convection was even eliminated at normal air pressure (760 Torr), and the maximum expected performance was achieved again (1). Yet, this new column hardware still required the use of cumbersome accessories such as vacuum pumps.
The first part of this article describes how a quasi-adiabatic thermal environment can be achieved for a standard chromatographic column free from any vacuum equipment. This new column assembly (or column hardware) is called the vacuum-jacketed column (VJC). In the second and third part of the article, it is reported how users can take advantage of the VJC in terms of i) easy column deployment to any optical detectors free from the cumbersome use of a column oven while still running the chromatographic analyses at high temperatures, and ii) improved LC–mass spectrometry (MS) performance when efficiently coupling (zero post-column dispersion) the mobile phase preheater, the chromatographic column, and the electrospray ionization probe for MS detection.
From the Impractical “Proofâof-Concept Adiabatic” Column to a User-Friendly VJC
The complex but ideal assembly required to prepare a chromatographic column under strict adiabatic environment has been described in previous works (1,2,10–12). The external surface area of the column and the internal surface area of the large internal diameter (6 cm) stainless steel vacuum chamber are first wrapped with aluminium foil to eliminate most of the heat transfer by electromagnetic radiation and the air pressure in the vacuum chamber was reduced to 10-5 Torr. Under such conditions, it was demonstrated experimentally that such adiabatic column hardware was fully successful, but it remained highly impractical for routine analyses (1,2,10–12).
In a first step towards designing a user-friendly adiabatic environment for the chromatographic column, 75% of the thermal mass at both the inlet and outlet SS endfittings of a 2.1 mm × 100 mm column was removed. In addition, two 2-cm i.d. disk-shaped PEEK side flanges (with circular rubber O-rings) were placed at the very ends of the column to seal it against a small 2-cm i.d. SS cylindrical vacuum chamber (still connected to a turbomolecular pump). This new design of the column hardware still ensures full adiabatic conditions and delivers maximum column performance (N = 33,800 at air pressure of 10-5 Torr) under severe chromatographic conditions (10 Watt/m viscous heating power). In contrast to the large 6-cm i.d. vacuum chamber, at atmospheric pressure or 760 Torr (N = 24,000), heat transfer by convection mode was eliminated for the small 2-cm i.d. chamber (N = 28,800) because the distance between the external column wall and the internal chamber wall is smaller than 1 cm (10,13). However, the success of the column assembly still relies on the presence of the cumbersome oil and turbomolecular vacuum pumps.
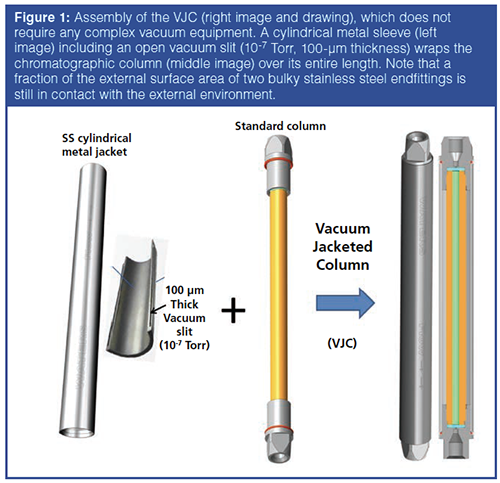
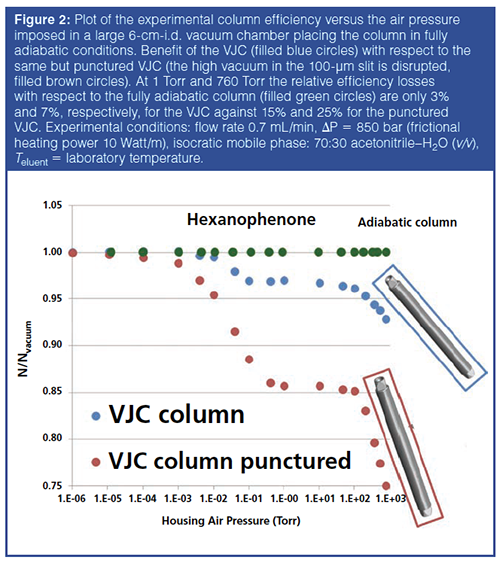
Therefore, in a second step, efforts were directed towards recreating a strict adiabatic environment for the column by getting rid of both the oil and turbomolecular vacuum pumps. As shown in Figure 1, this could be achieved by wrapping nearly the entire column length with a special cylindrical metal jacket. This jacket is less than 1-mm thick and includes across its thickness a thin (exactly 100-μm thick) open slit that spreads over its entire surface area. The metal jacket was supplied by Concept group, LLC. The thin metal sleeve was first annealed above its melting point in order to permanently remove the dissolved gases (H2) and a high vacuum (<10-5 Torr) was applied in the thin open slit, which was finally sealed. The metal jacket acts as a perfect thermal barrier between its internal and external volume space. The column is simply slit into the sleeve. The final columnâjacket assembly is the user-friendly VJC. Regarding performance, Figure 2 compares for the same compound (n-hexanophenone) the efficiency of the VJC to that of the same but punctured VJC (after drilling a hole through the entire thickness of the metal jacket in order to disrupt the high vacuum in the open slit and annihilate the effectiveness of the thermal barrier). Both the VJC and the punctured VJC were placed in the large internal diameter (6 cm) vacuum chamber and the air pressure was increased stepwise from 10-6 Torr to atmospheric pressure (1 atm). As expected, under extreme chromatographic conditions (flow rate: 0.7 mL/min, ΔP = 850 bar, eluent: 70:30 acetonitrile–H2O (v/v), heat friction power = 10 Watt/m), the efficiency of the punctured VJC drops by 14% from 10-6 Torr to 1 Torr (heat loss by diffusion mode through air being now permitted) and by another 13% from 1 Torr to 760 Torr (heat losses by both diffusion and convection modes being now permitted). In contrast, for the intact VJC column, these relative efficiency losses are measured at only 3% and 4%, respectively. In conclusion, the user-friendly VJC column is not operated under a strict adiabatic environment, but its performance remains very close to that of the strictly adiabatic but impractical column.
This is confirmed from infrared (IR) thermal images that show the difference in heat leaks between the VJC and the punctured VJC. Overall, for the sake of reproducibility, four different VJC columns (2.1 mm × 100 mm, packed with subâ2-μm particles) were embedded in the large (6-cm i.d.) vacuum chamber and their efficiency measured at air pressures of 760 Torr (heat can then be exchanged by convection and diffusion modes), 5 Torr (heat can be exchanged by diffusion mode only), and 10-5 Torr (heat can only be exchanged by residual electromagnetic radiations). The efficiencies were also measured when placing the VJC inside the oven of the UHPLC instrument (similar to 5 Torr air pressure in the vacuum chamber, heat can be only exchanged by diffusion). All the efficiency results were measured for six small analytes (uracil, acetophenone, propiophenone, butyrophenone, valerophenone, and n-hexanophenone). On average, the efficiencies of the four VJCs measured at 760 Torr (representing laboratory still-air thermal environment) are equal to 96% (column 1), 96% (column 2), 94% (column 3), and 97% (column 4) of the maximum theoretical efficiency (100% at 10-5 Torr air pressure). These results reveal that the user-friendly VJC operates under quasi-adiabatic conditions; the small relative loss (close to -5%) in column efficiency was a result of residual heat leaks at the hot column outlet endfitting, which is not fully insulated by the thin vacuum slit embedded in the metal jacket (Figure 1).
Easy Deployment of VJCs to Optical Detectors at High Temperatures Without Oven
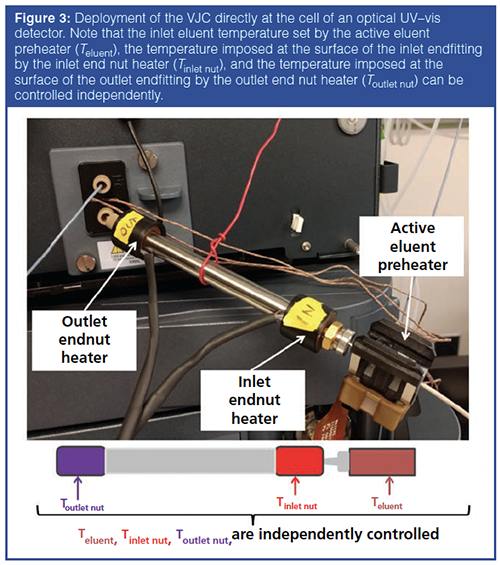
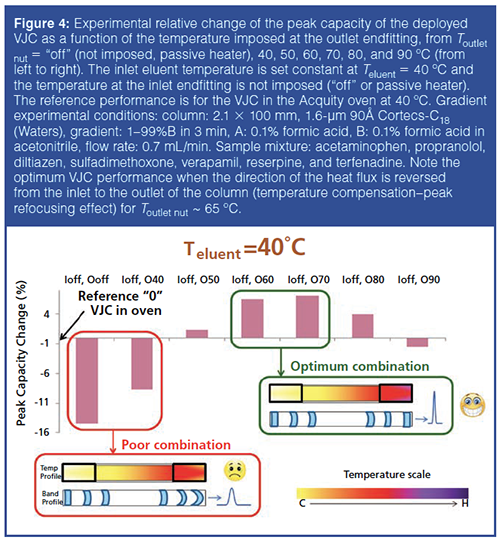
As demonstrated in the previous section, the VJC behaves nearly identically to the fully adiabatic column when the eluent temperature is set at room temperature. The heat leaks causing the 5% loss in column efficiency under extreme viscous heating conditions (10 Watt/m) are located at the outlet endfitting. Similar experiments were performed at elevated eluent temperatures (from room temperature to 90 ºC imposed by the active eluent preheater of the UHPLC instrument) while the VJC column was placed outside the column oven directly connected the inlet port of the UV detection cell (see Figure 3). Because the column is operated under quasi-adiabatic conditions, the use of a temperature oven is no longer required, and VJCs are then easily deployed directly to the inlet port of any detector (UV, refractive index [RI], fluorescence) while reducing post-column dispersion. The comparison between the gradient performances (peak capacity) of the VJC column (2.1 mm × 100 mm, packed with subâ2âμm particles, 0.7 mL/min, 1–99% acetonitrile gradient in 3 min) when it is deployed directly to the UV cell and when it is placed in the UHPLC oven was investigated at different temperatures of the entering mobile phase. Remarkably, despite a reduction in pressure drop and frictional heating, the performance of the deployed VJC is 2–15% lower than that of the same VJC in the instrument oven when increasing the inlet temperature from 25–60 ºC. This loss in gradient performance is now caused by the increasing intensity of the heat leaks from both the hot inlet and outlet endfittings of the deployed VJC to the cool laboratory air (the laboratory temperature is set at 25 ºC). A practical solution to this loss in gradient performance was proposed as shown in Figure 3: two small heaters were placed on the inlet and outlet end nuts of the column. The temperatures Teluent of the eluent entering the column (set by the active preheater of the UHPLC instrument), Tinlet nut of the inlet end nut (set by one small heater), and Toutlet nut of the outlet end nut (set by the second small heater) are controlled independently. For instance, Figure 4 shows the relative change in gradient peak capacity (with respect to that of the VJC placed in the oven, Teluent = Toven = 40 ºC, no heaters added) when keeping Teluent at 40 ºC, the inlet heater passive (“Ioff”), and stepwise increasing Toutlet nut from the laboratory temperature (“Ooff”) to 40, 50, 60, 70, 80, and 90 ºC. The experimental results reveal two poor Tinlet / Tinlet nut / Toutlet nut combinations: 40 ºC / off / off or 40 ºC. This can be easily explained from a qualitative viewpoint by estimating the intensity of the heat leaks at both column ends. The deployed VJC can be segmented into three zones: the inlet and outlet zones (about 1.5-cm-long) where heat leaks occur and the middle zone (7-cm-long for a 10-cm-long column), which is well thermally insulated. Accordingly, for the temperature combination 40 ºC / off / off or 40 ºC, the radial heat flux in both the inlet and outlet zones is oriented in the same direction: from the inlet column centre (40 ºC + ΔTinlet from frictional heating, yellow colour) to the laboratory air (25 ºC, white colour) and from the outlet column centre (40 ºC + ΔToutlet > 40 ºC + ΔTinlet from heat friction, red colour) to the laboratory air (25 ºC, white colour). As a result, the peak widths are adversely affected at both column ends (relative peak capacity losses of -15% and -9% for Toutletnut = off and 40 ºC, respectively). Note that in the thermally insulated middle zone, heat leaks are always negligible and they do not contribute much to peak width enlargement besides the usual and expected sample dispersion along packed beds in the absence of radial temperature gradients. In contrast, the temperature combinations 40 ºC / off / 60 ºC or 70 ºC (green colour frame) are very close to the optimum combination (40 ºC / off / 65 ºC) for a relative peak capacity gain of +7%; in this case, the heat flux direction is reversed from the inlet to the outlet zone. It is still from the inlet column centre, but it is now from the laboratory air (60–70 ºC, white colour) to the column centre (40 ºC + ΔToutlet < 60 ºC) at the outlet as a result of the “high enough” temperature applied by the heater to the outlet end nut. The initial peak deformation occurring at the inlet is then partially compensated at the column outlet. However, if Toutlet nut becomes too large (for example, 90 ºC), overcompensation occurs and the change in peak capacity returns to negative. This compensation phenomenon is similar to that previously reported in SFC using low-density mobile phases (carbon dioxide above 100 ºC, outlet pressure below 100 bar) where the temperature of the inlet eluent was controlled independently from that of the column oven (11).
Improved LC–MS Hyphenation with Zero PostâColumn Dispersion
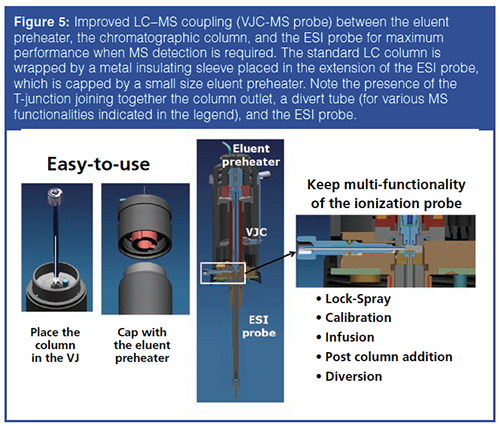
Most applications in LC analyses involve MS detection. The column outlet is usually connected to the ionization MS probe of the mass spectrometer. This causes post-column sample dispersion after the sample zone has eluted through long (up to 60–70 cm) and large internal diameter (100–125 μm) connecting tubes. This significantly affects the efficiency and gradient peak capacity of the column. For example, for a 2.1 mm × 100 mm column packed with sub-2âμm particles and a standard LC–MS interface (column → divert/infusion valve → ESI probe), the post-column sample dispersion is as large as 4.3 μL2 while the column dispersion (k = 1 at elution) is only 1.8 μL2. This means that only 30% and 55% of the expected column efficiency and peak capacity, respectively, can be observed after MS detection. A solution to that problem was proposed by coupling the chromatographic column directly to the ESI probe as shown in Figure 5. The conventional ionization probe is extended to a vacuum-jacket assembly, which accommodates the chromatographic column into the cylindrical insulating metal jacket. The assembly is then closed with a cap containing a newly designed eluent preheater. In addition, the MS probe conserves all its functionalities by designing a T-junction between the column outlet, the side tubing (used for MS lockâspray, calibration, direct infusion, make-up flow, and diversion), and the ESI probe tube.
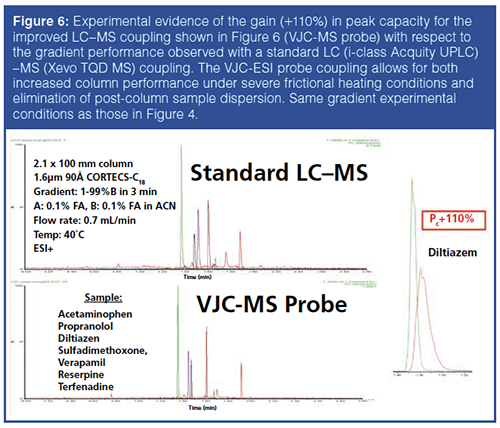
The proof-of-concept of this new research prototype LC–MS interface was tested regarding the separation of small molecules (acetaminophen, propranolol, diltiazen, sulfadimethoxone, verapamil, reserpine, and terfenadine) under challenging (10 Watt/m frictional heating) gradient chromatographic conditions (2.1 × 100 mm column, packed with sub-2-μm particles, gradient: 1–99%B in 3 min, A: 0.1% formic acid, B: 0.1% formic acid in acetonitrile, flow rate: 0.7 mL/min, inlet temperature 40 ºC, ESI+). The MS chromatograms recorded for the standard LC–MS system (standard column, 60 cm × 100 μm + 75 cm × 125-μm connecting tubes between the column outlet and the ESI probe) and the new VJCâESI probe interface are shown in Figure 6. The observed relative gain in peak capacity was +110%. This is illustrated in the peak shape of the compound diltiazem in the right insert in Figure 6. This relative gain is explained by i) the nearly complete elimination of the post-column dispersion up to the T-junction and ii) the improved gradient performance of the quasi-adiabatic VJC with respect to the standard column placed in a standard oven.
Conclusion
This article has demonstrated that it is possible to design an easy-to-use (regular size) column under quasi-adiabatic environment without depending on cumbersome vacuum accessories (oil pump, turbomolecular pump, stainless steel tubes, rubber O-rings), which make routine LC and SFC analyses impractically long. In order to achieve this, the chromatographic column is simply wrapped in a cylindrical insulating metal sleeve acting as a thermal barrier between its inside and outside faces: this defines the VJC.
Experimental results show that 95% of the maximum expected column efficiency is achieved when operating under intense frictional heating conditions (10 Watt/m) at room temperature. The 5% efficiency loss is caused by the residual heat leaks at both column ends. In addition, the VJC can be easily deployed to any optical detectors without highly dispersive post-column connecting tubes and the constraining presence of the column oven manager when operating the column at elevated temperatures. Performance even superior to that of the same VJC but placed in a conventional oven can be achieved by independently fixing the temperature of the inlet and outlet endfittings to sharpen and minimize the peak width at exactly the detection point.
Most importantly and for routine applications involving MS detection, a nearly zero-dispersion VJC-ESI probe interface was designed. The advantages of the VJC-ESI probe interface are twofold: i) maximum column performance of the VJC under extreme viscous heating conditions (as a result of the presence of the insulating metal sleeve) and ii) significant reduction of the post-column sample dispersion of a standard LC–MS system (by getting rid of unnecessarily long and wide internal diameter connecting tubes). Overall, the peak capacity of narrowâbore columns (2.1 mm × 100 mm long) packed with sub-2-μm particles is more than doubled with respect to the classical LC–MS configuration.
Acknowledgements
The author would like to thank Mike Fogwill, Martin Gilar, Jason Hill, Joseph A. Jarrell, Wade Leveille, and Joseph Michienzi (Waters Corporation, Milford, Massachusetts, USA) for their constant technical contributions, fruitful discussions, and suggestions for this research project.
References
- F. Gritti, LCGC North America36(s6), 18–23 (2018).
- F. Gritti, M. Gilar, and J. Jarrell, J. Chromatogr. A1456, 226–234 (2016).
- H.-J. Lin and Sc. Horvath, Chem. Eng. Sci.36, 47–55 (1981).
- F. Gritti and G. Guiochon, Anal. Chem.80, 5009–5020 (2008).
- F. Gritti and G. Guiochon, Anal. Chem. 80, 6488–6499 (2008).
- J. Kostka, F. Gritti, G. Guiochon, and K. Kaczmarski, J. Chromatogr. A1217, 4704–4712 (2010).
- K. Kaczmarski, F. Gritti, J. Kostka, and G. Guiochon, J. Chromatogr. A1216, 6575–6586 (2009).
- D. Poe and J. Schroden, J. Chromatogr. A 1216, 7915–7926 (2009).
- F. Gritti, M. Gilar, and J. Jarrell, J. Chromatogr. A1444, 86–98 (2016).
- F. Gritti, M. Fogwill, M. Gilar, and J. Jarrell, J. Chromatogr. A1468, 217–227 (2016).
- F. Gritti, M. Fogwill, M. Gilar, and J. Jarrell, J. Chromatogr. A1472, 107–116 (2016).
- T.L. Bergman, A.S. Lavine, F.P. Incropera, and D. Dewitt, Fundamentals of Heat and Mass Transfer, (John Wiley and Sons, Hoboken, New Jersey, USA, 7th ed., 2011).
- G.D. Raithby and K.G.T. Hollands, in Advances in Heat Transfer (Academic Press, New York, New York, USA, vol. 11, 1975), pp. 265–315.
Fabrice Gritti is a Principal Consulting Scientist at Waters Corporation, Milford, Massachusetts, USA. He received his Ph.D. in chemistry and physics of condensed matter from the University of Bordeaux in France, in 2001 and worked with Georges Guiochon as a research scientist until 2014 at the University of Tennessee Knoxville, USA. His research interests involve liquid–solid adsorption thermodynamics and mass transfer in heterogeneous media for characterization and design optimization of new liquid chromatography instruments and columns. He has made fundamental contributions to separation science with over 30 seminars and tutorials, 50 keynote lectures, and 270 peerâreviewed publications.

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Advances in Non-Targeted Analysis for PFAS in Environmental Matrices
March 27th 2025David Megson from Manchester Metropolitan University in Manchester, UK, spoke to LCGC International about the latest developments in non-targeted analysis (NTA) of per- and polyfluoroalkyl substances (PFAS) in environmental matrices based on a recent systematic review paper he has collaboratively published (1).










