Recovering from a COVID-19 Shutdown: Tips and Tricks for Starting Up, Part II
LCGC North America
Taking a systematic approach to restarting liquid chromatography instrumentation following the COVID-19 shutdowns will save money and time in the long run.
COVID-19-related laboratory shutdowns are sure to cause a myriad of problems with liquid chromatography (LC) instrumentation across the globe. Taking a systematic approach to restarting these systems will save money and time in the long run by preventing problems that may otherwise appear in days or weeks following startup.
In March of this year, many organizations took unprecedented steps to halt the spread of COVID-19, including severely restricting work in laboratories, or even shutting down entire laboratories, buildings, and worksites for weeks at a time. While some of these shutdowns were planned days in advance and executed well, I have heard many stories from scientists indicating that the shutdowns were sudden, and did not allow time to properly prepare their analytical instrumentation to be idle for weeks or months at a time. Unfortunately, this means that these scientists are going to encounter many challenges when they return to the laboratory that will necessarily include a lot of troubleshooting to figure out why their systems are not working properly before they can return to their normal experience of producing high quality data. For last month’s installment of “LC Troubleshooting,” I asked Tony Taylor to join me in pulling together advice for starting up liquid chromatography (LC) systems after they have been idled for weeks or months at a time (1). In that installment, we mainly addressed challenges with microbial growth in solvent bottles and different parts of the LC system itself, and obstruction of the LC flow path that can occur as a result of precipitation of buffer salts or other debris. For this month’s installment, I’ve asked Tony to join me again, this time sharing advice related to the health of columns, qualifying system performance, and a little about restarting work with LC-mass spectrometry (MS) systems in particular. I hope that these suggestions are helpful as you return to the laboratory, but I am sure many strange things will be observed after so many LC users have been away from the laboratory for prolonged periods. If you’ve encountered a problem and gained some troubleshooting experience that you think others might be able to learn from, please don’t hesitate to send your story my way.
The Column: Is It Still Okay?
Last month, we noted that the column is the heart of any chromatography system, and, as such, we need to ensure that our columns are in a healthy condition prior to performing analyses. It is possible that the shutdown period may lead to mechanical or chemical problems, and the solutions to these problems will be different. Until you know that the column has been properly flushed, the column outlet should not be connected to the rest of the system. Disconnecting the column outlet from the flow path will avoid any unwanted compounds or debris that may come out of the column from causing problems with any other components in the system. You can either attach a waste line to the column outlet to collect the effluent, or simply let it drip into a beaker or similar container.
Mechanical Problems
If your column was left on the system when the laboratory was shut down, there is a possibility that it will now be full of air, because the mobile phase solvent may have evaporated over time. Similarly, if the column was removed from the system, but not plugged at both ends, it will again be full of air, which can lead to problems for many types of columns. We need to carefully guard against applying high pressures to columns that are dry, as this may cause mechanical reorganization of the packed bed of particles, leading to unwanted voids, channeling, and significantly reduced column performance. In the following discussion, please bear in mind that the pressure applied to the column inlet should be increased gradually when first turning the flow back on (in steps of <10 bar where possible); this can be achieved by starting the pump at a very low flow rate (for example, 10 µL/min. for 2.1 to 4.6 mm i.d. columns), and increasing the flow rate in steps of 10 µL/min (larger steps can be used provided the corresponding pressure increase is not much more than 10 bar per step). Some newer systems also provide the option to specify a flow rate ramp rate that is used when the pump is turned on.
It may be possible that, upon starting the column flushing procedure, a high-back pressure is encountered due to blockages in either the column inlet frit (pressure increase will be immediately noticeable) or the outlet frit (pressure will build more gradually over time). In the case of the former, it may be possible to reverse the direction of the column prior to turning on the flow, in order to back-flush the debris from the frit. Overall, this may have the longer-term effect of slightly reducing the efficiency of the packed bed, but the column should be usable for your application, unless it was heavily voided prior to the instrument shutdown.
Chemical Problems
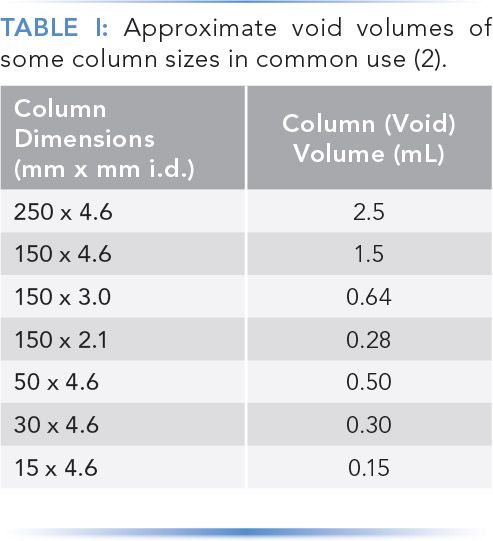
In most cases, the following column flushing routine can be applied to reversed-phase, silica-based stationary phases as a kind of generic column cleanup step. Approximate column volumes for several different dimensions of columns in common use are given in Table I.
- Set the column thermostat compartment to 60 °C.
- Flush with the following solvents in the order shown. For 2.1 mm i.d. columns use a flow rate of about 0.1 mL/min; for 4.6 mm i.d. columns use a flow rate of about 0.5 mL/min.
- 10:90 methanol:water for 20 column volumes (to remove any
- precipitated buffers)
- Increase organic composition to 100% methanol, and flush for 20 column volumes.
- Flush with 20 column volumes of 75:25 acetonitrile:isopropyl
- alcohol (IPA).
- Flush with 20 column volumes of the starting mobile phase of your method.
The rationale behind this series of flushing solvents is to cover a wide range of solvent polarities that give the best chance of dissolving and eluting anything that has adsorbed to the stationary phase. Only after these flushing steps should the column be reconnected to the detector. If the column flow direction has been reversed as discussed above, switch back to the original orientation prior to reconnecting the column to the system.
In this brief discussion, we have focused on suggestions for restarting work with reversed-phase columns, and in a very generic way. Readers interested in a more detailed discussion of cleaning procedures for reversed-phase columns are referred to the excellent LCGC North America article on this topic by Ron Majors (2).
Is It Safe to Collect Data Again?
At a minimum, before collecting important data again, one should run the system suitability test that is appropriate to the analysis at hand. The topics of system suitability and operational qualification (OQ) have been discussed in prior installments of “LC Troubleshooting” by John Dolan, and readers interested in more detail are referred to these articles (3,4).
In general terms, system suitability tests are designed to provide data that indicate an LC system is functioning in a way that it can be expected to produce reliable data for a particular analysis. In other words, it is application- or method-specific. In many situations, these data are sufficient to give the user confidence that the system is “ready to go.” However, in other situations and under certain regulatory frameworks, it may be necessary to carry out a more extensive OQ of the instrument that will verify instrument performance, regardless of the methods being run. While doing OQ tests will undoubtedly take more time when restarting an LC system, we feel strongly that this time spent at startup can save a lot of time in the long term by catching problems early, and addressing them before data acquisition resumes. In other words, a little extra effort now will decrease the likelihood that we encounter unwelcome surprises down the line!
A typical OQ verification routine will include some or all of the following tests that we have annotated briefly to give an explanation of what the test does, and the information it provides toward an assessment of operational performance. Wherever a “reference compound” is referred to in these descriptions, caffeine is very commonly used, particularly for systems with ultraviolet (UV) detectors.
Pump Flow Rate Accuracy and Precision
Typically, a number of flow rate measurements are made at different flow rates using a digital flow meter. Flow rate accuracy is important for transfer of methods between systems and retention time agreement with standard methods of test. Flow rate precision has a direct impact on the repeatability of peak height and area.
Column Temperature Accuracy and Stability
Typically, a temperature sensor is used to measure either the column compartment temperature, or the temperature of the column effluent. Temperature is measured over time at two different setpoints, and the absolute difference between them, as well as the variability, are compared to manufacturers or regulatory criteria. Temperature accuracy can be critical for chromatographic selectivity, especially when separating ionizable analytes (5), and temperature stability has a strong influence on the repeatability of retention times.
UV Wavelength Accuracy
Typically, a caffeine or holmium oxide standard is used under conditions of no mobile phase flow, and the absorbance is recorded at several wavelengths across the range of the detector. The measured positions for the peaks (maxima) and troughs (minima) of the standard are compared to the known (expected) values for the standard. The difference between the measured and expected wavelengths of maximum and minimum absorption is compared to manufacturers specifications. Wavelength accuracy is vital for both qualitative and quantitative work, and transferability of methods between systems.
Detector Noise and Drift
Short- and long-term variation in the detector signal is determined with a fixed eluent composition (typically water). The detector signal is measured over a certain period of time and at a particular frequency to determine the short-term variation in the signal, referred to as the noise. The slope of the detector signal over a longer time period (tens of minutes) is also measured, and this is referred to as the detector drift. The magnitudes of the noise and drift directly influence the ability to differentiate between real peaks for low concentration analytes and random variation in the detector signal.
Signal-to-Noise Ratio (S/N)
The detector sensitivity is also evaluated using a reference compound under specific conditions and compared to a target specification. There are many different manufacturer and regulatory recommendations on the topic of signal-to-noise ratio (S/N), and you should follow the guidance most appropriate for your situation.
Injection Precision
Using a reference standard, the peak height and area are measured for several replicate injections, sometimes for both small and large injection volumes. The absolute values for peak height and area, as well as the relative standard deviation of each value, are compared to manufacturers specifications. Injection precision is particularly important for quantitative analysis; the better the injection precision is (that is, smaller standard deviation), the better is the ability of the method to differentiate between samples having similar analyte concentrations.
Detector Response Linearity
Typically a reference standard is injected multiple times in a range of concentrations that cover the normal operating range of the detector (for example, up to 1.5 AU for a UV detector). Statistical assessment of linearity is performed using a combination of linear regression, residuals analysis, F-tests, and relative standard deviation of detector response for each analyte concentration, as well as ratios of signals. Linearity of detector response is critical for quantitative analysis, and has a direct impact on the accuracy of analyte concentrations reported based on use of calibration curves.
Solvent Gradient Composition
Typically, a tracer compound such as acetone is added to one of the mobile phase solvents (usually the “B solvent”), and a method is used that steps through different mixtures of two solvents, one of which contains the tracer compound that can be observed by the detector (for example, acetone absorbs well at 265 nm). For example, a method may start at 0% B, and increase in steps of 5% B until 100% B is reached. The signal due the presence of the tracer compound is used as an indirect measure of the ratio of the volumes of the A and B solvent that are combined by the pump to make the mobile phase mixture of A and B. The average detector signal at each % B level is compared to the expected value, and the short-term variation at each step may also be evaluated. Finally, some OQ routines call for the analysis of a linear gradient profile using the same tracer compound. The accuracy and repeatability of the gradient profile are critical for both qualitative and quantitative analysis, transfer of methods between instruments, and repeatability of retention times.
Suggestions for LC Systems with Mass Spectrometric (MS) Detectors
MS detectors can be particularly susceptible to problems on startup following extended periods in standby mode or shutdown, and one needs to pay particular attention to these detectors prior to restarting work with them. As there is a lot of variation in maintenance protocols for different MS manufacturers and instrument types, it is essential that you carefully follow the manufacturers guidance when considering the following steps.
First, thoroughly clean the ionization source, preferably when the instrument is not under vacuum. However, it is not necessary to vent the instrument if it is under vacuum at the time of cleaning. In any case, follow the manufacturers recommended procedure for cleaning the source. The emitter (that is, sprayer or nebulizer) should also be checked carefully prior to re-establishing flow from the LC system, as residual eluent solvents evaporate during extended storage periods, often leaving residues or even blocking the flow path entirely, and can be difficult to remove. If significant residue or an obstruction is observed, remove the nebulizer and sonicate for 10 min (first in water, and then in IPA). Be careful to suspend the nebulizer tip in the cleaning solvent in such a way that it does not contact the bottom or walls of the container, as this could damage the tip itself, and affect spray performance.
If the instrument has been vented, carefully monitor the vacuum levels in the instrument when pumping it back down, and check the vacuum levels against the manufacturers specifications.
Before using the MS for data acquisition a verification of its performance will be required. At a minimum this will include a full tune (autotune), and this is typically achieved using the manufacturer’s recommended tuning compound or solution and performance criteria. If your instrument includes an on-board tuning solution that can be activated using the control software, make sure there is enough tuning solution in the reservoir prior to starting the autotune routine. The autotune routine will tune the electrostatic lenses within the instrument as well as the voltages applied to the mass filtering device in order to optimize and verify, amongst other things, mass accuracy across a wide range of values, instrument sensitivity, and response profile.
While the on-board autotune is very useful to set the detector parameters and check them against the manufacturer’s performance requirements, one may also need to carry out further “whole-system” performance checks with a typical set of performance criteria involving: 1) response linearity (or response profile if it is expected to be non-linear based on previous experience); 2) injection precision; 3) carryover; 4) signal-to-noise ratio; and 5) minimum detection limits.
Summary
Given the variety of ways different laboratories were shutdown early on in the global COVID-19 outbreak, it is likely that LC users will encounter a wide variety of problems when they return to their laboratories and resume work with their instruments. In this installment of “LC Troubleshooting,” we have provided suggestions specific to handling LC columns and LC systems with MS detectors when restarting work after a long time away. We have also discussed the value of running system suitability and OQ tests before starting to collect important data again. These tests will be helpful for identifying problems that may have been caused by the shutdown, so that they can be resolved before causing trouble later on.
References
- D.R. Stoll and T. Taylor, LCGC North Am. 38(6), 320–324 (2020).
- R.E. Majors, LCGC North Am. 21(1),19–26 (2003).
- J.W. Dolan, LCGC North Am. 22(5), 430–435 (2004).
- J.W. Dolan and G. Hall, LCGC North Am. 20(9), 842–848 (2002).
- D.R. Stoll, LCGC North Am. 38(5), 261–268 (2020).

Dwight R. Stoll is the editor of “LC Troubleshooting.” Stoll is a professor and the co-chair of chemistry at Gustavus Adolphus College in St. Peter, Minnesota. His primary research focus is on the development of 2D-LC for both targeted and untargeted analyses. He has authored or coauthored more than 60 peer-reviewed publications and four book chapters in separation science and more than 100 conference presentations. He is also a member of LCGC’s editorial advisory board. Direct correspondence to: LCGCedit@mmhgroup.com

Tony Taylor is the Chief Science Officer of Arch Sciences Group and the Technical Director of CHROMacademy. Direct correspondence to: LCGCedit@mmhgroup.comLCGCedit@mmhgroup.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.
Troubleshooting Everywhere! An Assortment of Topics from Pittcon 2025
April 5th 2025In this installment of “LC Troubleshooting,” Dwight Stoll touches on highlights from Pittcon 2025 talks, as well as troubleshooting advice distilled from a lifetime of work in separation science by LCGC Award winner Christopher Pohl.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.









