High-Throughput and High-Efficiency Separations of Antibodies by Reversed-Phase Chromatography Using Organic Alcohols
LCGC North America
The strategies described here can improve the efficiency and analyte recovery of chromatographic methods used to characterize monoclonal antibodies and antibody– drug conjugates.
Biomacromolecules, especially monoclonal antibodies, are complex molecular species that have a myriad of functional groups. This complexity has led to much discussion on strategies for improving efficiency and analyte recovery of chromatographic methods used to characterize these molecules. This edition of “Column Watch” will focus on the roles of temperature and organic modifier for yielding improved efficiency in analyzing biomacromolecules. It is shown that these variables can be used together to achieve higher throughput, resolution, and recovery when analyzing monoclonal antibodies and antibody-drug conjugates.
Monoclonal antibodies (mAbs) are a promising class of biologics for the treatment of several autoimmune diseases and cancers. An additional application of mAbs, however, is when a cytotoxic payload (such as a drug) is attached to the mAb, allowing for the mAb to target a certain cell type or tissue and deliver the payload to a specific target. This combination of mAb plus cytotoxic drug, connected through an organic linker, is known as an antibody-drug conjugate (ADC). As of January 2020, there are 89 ADCs in the pharmaceutical pipeline (1).
One downside of mAb-based drugs, however, is that, due to their structural complexity, there is significant heterogeneity. This heterogeneity can arise due to the presence of charge variants, glycosylation variants, or phosphorylation variants; these arise by nature of the biological production process.
Several different chromatographic strategies are applied to investigate and resolve the structural and chemical variants of mAbs. Size-exclusion chromatography is used as one method to assess the aggregation of a mAb sample. Ion-exchange chromatography is a suitable method to conduct charge variant analysis. Both chromatographic modes can present issues of compatibility with electrospray ionization mass spectrometry (ESI-MS), which is routinely used for protein characterization. Hydrophobic interaction chromatography (HIC) is routinely employed for analysis and characterization of protein glycans. Reversed-phase chromatography has long been a method of choice for analyzing proteins due to its high resolution and compatibility with mass spectroscopy (MS). Reversed-phase chromatography of proteins, however, has its own issues. Of primary significance is that protein structures can be flexible in comparison to structures of small organic molecules. This fact may present a chromatographic challenge, as various structural conformations may differentially interact with the stationary phase. With proteins, peak shape in reversed-phase chromatography is generally enhanced by parameters that stabilize a single denatured state (2–4). Temperature is one parameter that can dramatically affect the tertiary and quaternary structure of proteins, and could lead to a “denatured state.”
Another aspect of protein and peptide reversed-phase chromatography is that, for most applications, elution must be by utilization of a solvent strength gradient. This requirement is due to at least two reasons: 1) polypeptides are generally polyionic, and, therefore, can present problems of secondary interactions with the silica surface, potentially causing issues of peak tailing; and 2) partitioning of polypeptide analytes between the mobile and stationary phase occurs over a narrow window of solvent strengths (as compared to most small molecules), therefore exhibiting much more of an on-off adsorption phenomenon. With the requirement for gradient elution comes the requirement for column re-equilibration prior to injection of a sample. Column re-equilibration can be sped up by reducing changes to the solvation state of the silica surface, as has been shown by inclusion of low levels of small, primary alcohols in the mobile phase (5,6). How this mechanistically takes place has not been defined, but computer modeling of short, primary alcohols in binary mobile phase systems is consistent with intercalation of the alcohol into the stationary phase, with the hydroxyl hydrogen-bonding to the surface silanols or an adsorbed water layer (7). This phenomenon may have additional benefits in masking silanols, therefore improving peak shape. Scott and Simpson reported that 1-butanol can form a simple monolayer on a C18-bonded silica surface; this also fits with a model of a small, primary alcohol hydrogen bonding to the surface silanols (8).
High temperature has been shown to be necessary in achieving optimal analyte recovery and peak shape of mAbs in reversed-phase chromatography (9). This fact has been confirmed to be the case, irrespective of the column used or the specific mAb sample (10). Additionally, Fekete and associates have shown that inclusion of low levels of 1-butanol reduced the temperature optimum for the reversed-phase chromatography of the mAb (9). Thus, the mechanism of any conferred benefits of inclusion of low levels of primary alcohols in the mobile phase is not entirely clear. These previously published data suggest a primary mechanism of masking of the silica surface; another possibility might be imagined to explain the effects on antibody chromatography in which the intercalated 1-butanol is oriented with the hydroxyl facing the bulk mobile phase, thus lending some polarity to the environment at the antibody-stationary phase interface. Such models might be elucidated by inspecting results with analogs of 1-butanol.
Effect of Organic Alcohol on Monoclonal Antibody Characterization
These ideas were investigated further using a commercially available mAb reference material. Initially, the goal was to at least confirm previous reports on the chromatographic effects of 1-butanol on the reversed-phase chromatography of mAbs. Two primary alcohols, 1-propanol and 1-butanol, were investigated. Figure 1 shows chromatographic results of the recovery of the mAb at varying percentages of 1-butanol at 55 °C.
Figure 1: Analysis of mAb reference
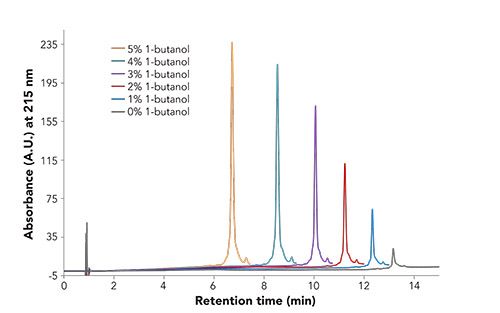
material by reversed-phase chromatography with varying amounts of 1-butanol. Conditions: Column: BIOshell A400 Protein C4, 10 cm x 4.6 mm i.d., 3.4-µm; Mobile Phase: [A] 70:30 0.1% TFA in water: 0.1% TFA in acetonitrile; [B] 60:40 0.1% TFA in water: 0.1% TFA in acetonitrile; [C] 70:25:5 0.1% TFA in water: 0.1% TFA in acetonitrile: 0.1% TFA in alcohol; [D] 60:35:5 0.1% TFA in water: 0.1% TFA in acetonitrile: 0.1% TFA in alcohol; Gradient: For x = 0, 20, 40, 60, 80, or 100; [(100-x)% A, 0% B, x% C, 0% D] to [0% A, (100-x% B), 0% C, x% D] in 15 min; Flow Rate: 1.0 mL/min; Column Temp.: 55 °C; Detector: UV, 215 nm; Injection: 3.0 µL; Sample: mAb reference material, 1 g/L, 0.05% TFA in water.
As noted in Figure 1, the peak area and height of the analyte increased as the concentration of 1-butanol increased. In addition, as the concentration of 1-butanol increased, one can begin to resolve impurities from the main analyte peak. Finally, the retention time of the mAb decreased with increasing 1-butanol concentration. This phenomenon was further investigated by looking at how temperature played a role in the recovery of the mAb. Figure 2 displays the results of this analysis.
Figure 2: Recovery, measured by peak area, as a function of temperature and 1-butanol concentration. Note that the maximum recovery of the analyte occurs at much lower temperatures as the concentration of 1-butanol increases. Chromatographic conditions are the same as those described in Figure 1 except for temperature, which was varied as indicated in the figure.
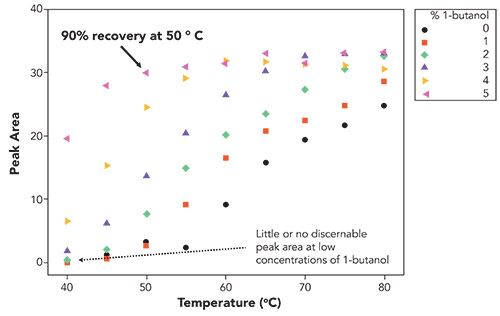
As can be seen in Figure 2, it should become obvious that one of the main advantages of including 1-butanol in the mobile phase is the much lower temperature required to achieve maximum recovery of the analyte. The data, however, cannot differentiate effects due to possible mitigation against thermal degradation or impacts on the actual chromatography of the mAb. The experiment was repeated, this time with 1-propanol as the mobile phase modifier. Figure 3 shows the results of this analysis.
Figure 3: Recovery, measured by peak area, as a function of temperature and 1-propanol concentration. Note the much-reduced recovery at 50 °C with 1-propanol versus 1-butanol. Chromatographic conditions were the same as those described in Figure 1 except for temperature which was varied as indicated in the figure.
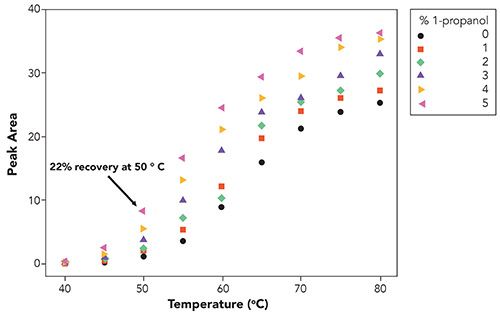
As shown in Figure 3, the temperature required to achieve the maximum recovery of the analyte was much higher (around 80 °C) in comparison to 1-butanol. While 1-propanol has been shown to have similar benefits as 1-butanol for keeping the silica surface solvated, and shielding surface silanols, it clearly did not provide the same chromatographic benefits in this case, as compared to 1-butanol (5,6).
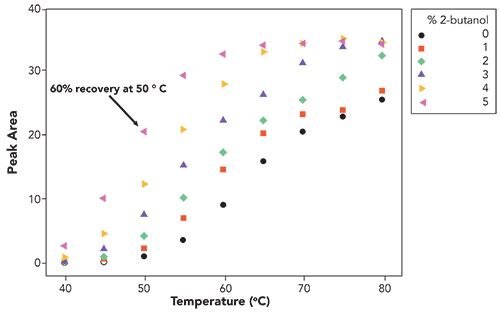
Continuing the investigation, the effect of type of alcohol (primary, secondary, and so forth) on analyte recovery was examined. As noted in Figure 4, the secondary alcohol elicits good recovery of the antibody, albeit not as good as 1-butanol. It could be inferred that, due to steric effects, 2-butanol should be not as effective as primary alcohols in hydrogen-bonding with surface silanols or an adsorbed water layer. However, 2-butanol achieved higher recovery of the analyte at low to moderate temperatures than with 1-propanol, suggesting that the mechanistic explanation is not as simple as hydrogen-bonding to the silica surface.
Figure 4: Recovery, measured by peak area, as a function of temperature and 2-butanol concentration. Chromatographic conditions are the same as those described in Figure 1 except for temperature which was varied, as indicated, in the figure.
A final test was to examine the use of 1,4-butanediol. The idea is that this alcohol could possibly play a dual role of hydrogen-bonding to the silica surface, as well contributing additional polarity to the interface where protein adsorption takes place on the stationary phase. The results are shown in Figure 5.
Figure 5: Recovery, measured by peak area, as a function of temperature and 1,4-butanediol concentration. Note that the concentration of 1,4-butanediol does not appear to influence recovery. Chromatographic conditions are the same as those described in Figure 1, except for temperature which was varied, as indicated, in the figure.
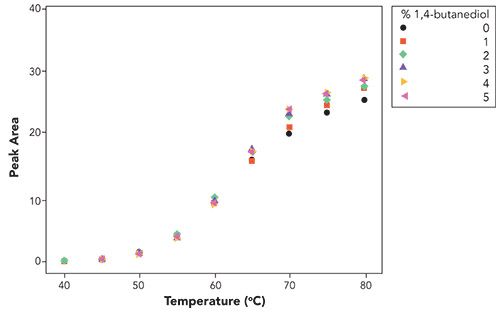
As can be deduced from Figure 5, there appears to be no advantage in adding 1,4-butanediol to the mobile phase. Perhaps the two terminal hydroxyls render it sufficiently polar such that it no longer readily distributes within the stationary phase to provide any performance benefit to the reversed-phase chromatography. In fact, the collective results beg the question, could the difference in the effects simply be due to the solubility of the alcohol within the stationary phase?
It also suggested to the authors that it would be most interesting to do a comparative study with an identical column or particle, but with a bonded butanol phase (the terminal hydroxyl exposed to the bulk solvent). Such an experiment could shed light on this mechanistic question regarding the conferred impacts of added butanol in the mobile phase. As mentioned earlier, perhaps the hydroxyl faces the bulk mobile phase to lend polarity to the environment at the antibody-stationary phase interface.
Effects of 1-Butanol on Analysis of an ADC
In addition to all the inherent complexity associated with a mAb, characterization of an ADC introduces further complexity to the separation challenge due to the addition of the cytotoxic drug (payload) and organic linker. Creation of a cysteine-linked ADC, where the payload is attached to cysteine amino acid residues, leads to a mixture of ADC molecules with different amounts of payload attached to the mAb vehicle. Commonly seen permutations of a cysteine-linked ADC molecule include the native mAb molecule (no payload attached to the mAb), followed by ADCs with two, four, six, or eight payloads attached. In addition, impurities from the mAb production process and unbound cytotoxic drug are also present in ADC samples.
As was discussed in the previous section, incorporating 1-butanol into the mobile phase yielded improved recoveries, higher throughput, and higher efficiency than with mobile phases without 1-butanol. To see if this observation can be translated to a more complex protein molecule, 1-butanol was incorporated into the mobile phase used for the analysis of an ADC by reversed-phase chromatography. Figure 6 displays the chromatographic results of this analysis. Note the improved resolution and recovery of some of the peaks corresponding to labeled heavy and light chains of the ADC. Interestingly, there does not appear to be a reduction in retention time as was seen with the mAb analyte.
Figure 6: Analysis of an ADC by reversed-phase chromatography (a) with or (b) without 1-butanol. Conditions: Column: BIOshell A400 Protein C4, 10 cm x 4.6 mm I.D., 3.4-µm; Mobile Phase: [A] 0.1% DFA in water; [B] 0.1% DFA in acetonitrile; [C] 95:5 0.1% DFA in acetonitrile: 0.1% DFA in 1-butanol; Gradient: 25% B to 45% B in 10 min (no butanol method) OR 25% C to 45% C in 10 min (with butanol); Flow rate: 0.5 mL/min; Column temp.: 55 °C; Detector: UV, 215 nm; Injection: 2.5 µL; Sample: ADC SR-388, 1 mg/mL, 0.1% DFA in water.
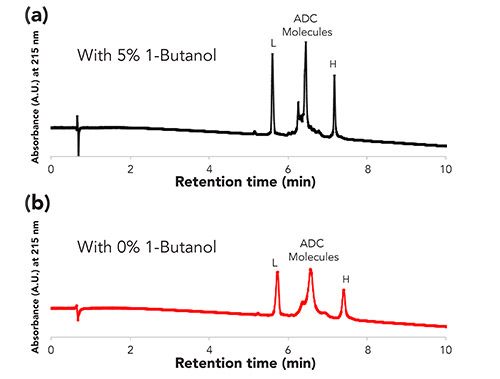
However, there is an effect on the separation as the efficiency of the method with 1-butanol was higher (as indicated by more narrow peak widths) than without the 1-butanol. An explanation for this result may possibly be tied with the tertiary structure of the protein. The structural integrity of cysteine-linked ADCs is less than in native mAbs, due to some of the cysteines used in disulfide bonds between the light and heavy chains of the mAb being reduced to add the payload molecules. Since the method conditions would permit denaturing of the protein, less energy would be required to cause denaturation. Denatured proteins expose more hydrophobic amino acids to the stationary phase, and thus could lead to peak broadening due to a stronger interaction between the stationary phase and the protein. Perhaps the 1-butanol is solvating the protein molecule and minimizing protein denaturation (or minimizing the number of denatured forms of the protein) leading to sharper peaks. Additional investigations are needed to fully test these possible explanations.
Conclusions
Despite these seemingly conflicting results, what emerges is a picture in which the role of the alcohol in conferring a chromatographic benefit to the reversed-phase chromatography of mAbs and ADCs (or maybe most any other immunoglobulin G molecule) is not as simple as has been suggested from other chemical and computer modeling studies-that something other than masking the silica surface is at play here. Perhaps there is a separate effect on the thermal stability of the mAb in this common mobile phase. Nevertheless, as shown in Figures 1 and 2, the addition of 1-butanol to the mobile phase can elicit excellent recovery of an antibody standard at far lower temperatures than in its absence, and can serve as a general method for reversed-phase chromatography of antibodies, or perhaps any other proteins that exhibit poor peak shape at moderate temperatures.
References
- A. Keown, Biospace https://www.biospace.com/article/with-3-approvals-in-2019-companies-see-great-future-for-adc-therapies/ Accessed June 6, 2020.
- K.D. Nugent, W.G. Burton, T.K. Slattery, B.F. Johnson, and L.R. Snyder, J. Chromatogr. A 443, 381–397 (1988).
- K. Benedek, S. Dong, and B.L. Karger, J. Chromatogr. 317, 227–243 (1984).
- X.M. Lu, K. Benedek, and B.L. Karger, J. Chromatogr. A 359, 19–29 (1986).
- L.A. Cole and J.G. Dorsey, Anal. Chem. 62, 16–21 (1990).
- A.P. Schellinger, D.R. Stoll, and P W. Carr, J. Chromatogr. A 1192(1), 54–61 (2008).
- J.L. Rafferty, J. Ling Zhang, I. Siepmann, and M.R. Schure, Anal. Chem. 79(17), 6551–6558 (2007).
- R.P.W. Scott and C.F. Simpson, Faraday Symp. Chem. Soc. 15, 69–82 (1980).
- S. Fekete, S. Rudaz, J.-L. Veuthey, and D. Guillarme, J. Sep. Sci. 35(22), 3113–3123 (2012).
- R. Eksteen, D. Bell, and H. Brandes, “Using Temperature to Improve Peak Shape of Hydrophobic Proteins in Reversed-Phase HPLC.“ HPLC Conference Poster (2014).
About the Authors

Cory E. Muraco is the Global Franchise Product Manager, Liquid Chromatography Technology, at MilliporeSigma in Bellefonte, Pennsylvania. Cory completed his graduate studies in 2013 at Youngstown State University where he focused on using analytical chemistry to deduce the role of protein primary and tertiary structure on protein oxidation. Cory joined Merck KGaA in 2013, where he assumed the role of R&D Scientist working in the Chemical Standards division developing certified reference materials (CRMs) for multiple industries. In 2015, Cory joined the HPLC Application and R&D division of Merck KGaA where he researched and developed new column technology and applications. In 2019, Cory was promoted to his current role, where he is responsible for developing and executing the marketing and R&D strategy surrounding new HPLC column technology.

Hillel K. Brandes is an Analytical Technology Specialist with MilliporeSigma in Salt lake City, Utah. In his prior role, he was a Principal R&D Scientist, focusing on HPLC product development and application support.
About the Column Editor

David S. Bell is a director of Research and Development at Restek. He also serves on the Editorial Advisory Board for LCGC and is the Editor for “Column Watch.” Over the past 20 years, he has worked directly in the chromatography industry, focusing his efforts on the design, development, and application of chromatographic stationary phases to advance gas chromatography, liquid chromatography, and related hyphenated techniques. His main objectives have been to create and promote novel separation technologies and to conduct research on molecular interactions that contribute to retention and selectivity in an array of chromatographic processes. His research results have been presented in symposia worldwide, and have resulted in numerous peer-reviewed journal and trade magazine articles. Direct correspondence to: LCGCedit@mmhgroup.com

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.












