Recent Advances and Applications of Passive Sampling Devices
Passive samplers have been developed in many different forms and used in different fields of study because of their unique capabilities. One part of recent reports has focused on benefiting from the advantages of passive sampling in areas such as wastewater-based epidemiology and non-targeted analysis. The other part mainly deals with novel approaches to improve the reliability and efficiency of the sampling process. This paper reviews major advances and new applications of this sampling strategy based on recently published scientific publications.
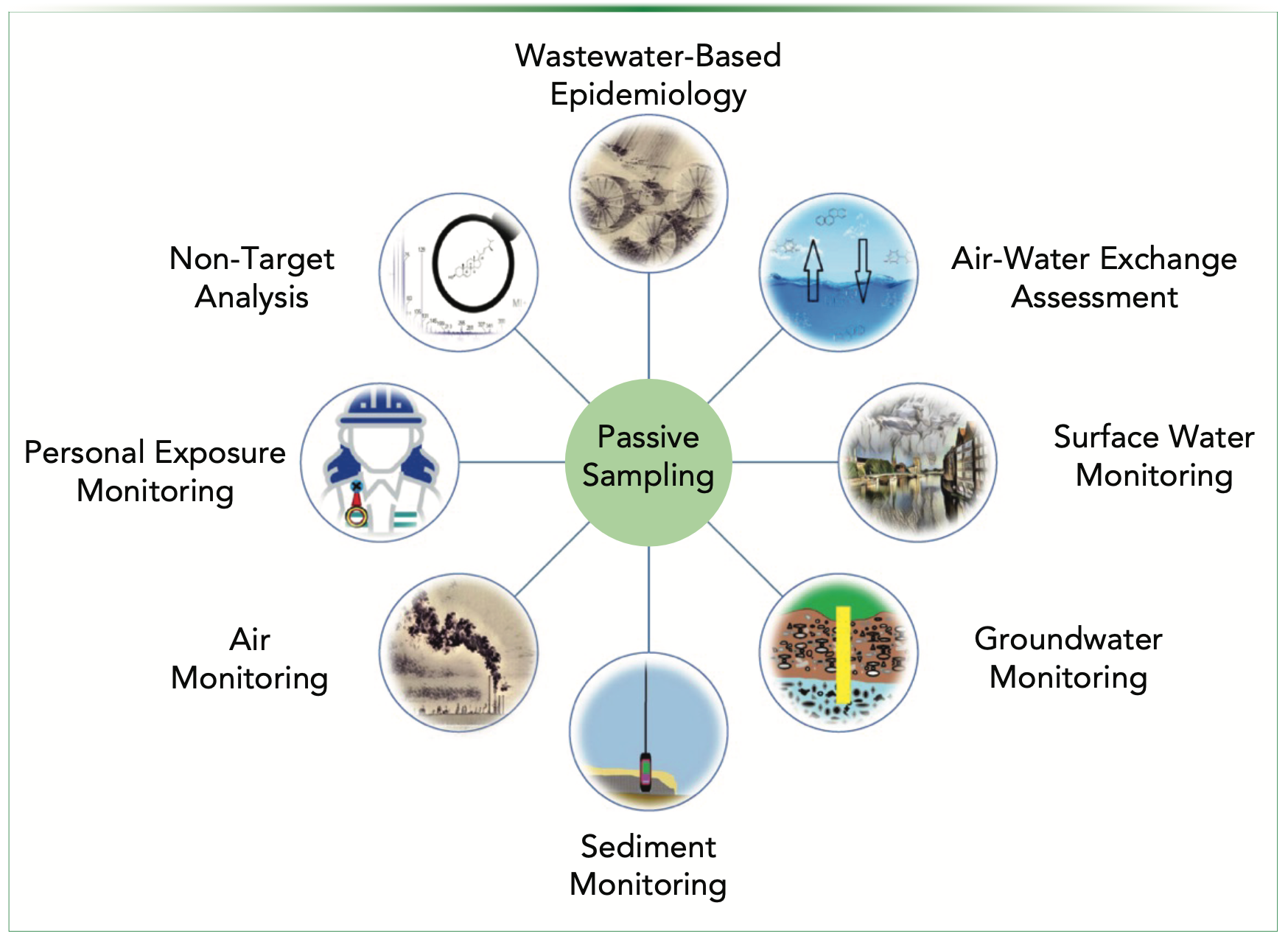
Among the common sampling strategies, that is grab, composite, and passive sampling, the latter has some unique features that have made it a useful tool, especially for environmental studies (1). Passive sampling can provide reliable data on the time-averaged concentration of contaminants. Temporary contamination events, such as provisional discharges and accidental spills which are not normally captured by other sampling strategies, could be recorded (chemically) by the passive sampler. In addition, this approach can enrich the trace contaminants over a rather long exposure and also provide toxicologically relevant data on bioavailable concentrations of the contaminants (2–4). Passive sampling is also possible and useful for the study of atmospheric organic contaminants, in similar approaches (of course taking into account the differences in receiving phases, sampler design, and analytes) (5). Despite its advantages, passive sampling needs a more complicated calibration procedure in order to produce valid quantitative data. This could be done using theoretical estimation of the partition coefficients of the analytes between the sorbents and the sampled media, considering its physicochemical properties (6). Another strategy (considered to be more reliable) is to calibrate the passive sampler in the real environment by comparing its analytical results with those of active sampling (7). In the following section, the most recent applications of various passive sampling devices will be briefly reviewed (Figure 1).
FIGURE 1: Recent application fields of passive sampling.
Applications and Developments of Passive Sampling
Since the first reports on quantitative applications of passive sampling for the determination of sulfur dioxide in air in 1973, diverse sorption phases and sampler designs have been reported for studying water and air contamination. Among them, semi-permeable membrane devices (SPMDs) (8), low-density polymer devices (LDPE) (9) providing higher surface areas and reduced consumable costs (10), silicon rubber (SR), Chemcatcher (11), the polar organic chemical integrative sampler (POCIS) (12), and solid-phase microextraction (SPME) (13) have been implemented for water sampling purposes. Regarding atmospheric contamination, SPMDs, similar to what is being used for water sampling, with various sorbing phases (14–16) have been utilized. Furthermore, devices based on porous materials have been developed for different semi-volatile compounds in the atmosphere, using for example polyurethane foam (PUF) (17), naphthylisothiocyanate (XAD) (18), and activated carbon (19) as sorbents.
Wastewater-based epidemiology (WBE) is one of the most recent application fields of passive sampling. In this field of study, untreated wastewater is collected and analyzed for the content and variations of health-related compounds (biomarkers), and the data are compared with the public health situation of the catchment (20). Obviously, grab sampling could only provide a snapshot of the wastewater under study, while the composition of the media is continuously changing due to the population routines as well as the social and climatic conditions, such as working days and holidays, vacation periods, seasonality, and atmospheric precipitations. Composite sampling, as it is conventionally used, could resolve such problems, but its implementation requires higher costs of operation and maintenance and also could produce large volumes of samples to be handled. Alternatively, passive sampling could significantly reduce cost and labor and at the same time eliminate or simplify many sample pretreatment steps, such as preservation, storage, filtration, extraction, and preconcentration.
One of the most recent applications of passive sampling in WBE studies is the investigation of the spread and dynamics of the SARS-CoV-2 virus (21). In this work, authors deployed polyethylene plastic strips, as simple forms of the sorbing phase, cotton cloth sheets, and unraveled polypropylene plastic ropes to sample the wastewater. The samplers then were transferred to the laboratory to extract and analyze SARS-CoV-2 RNA. The results suggested that considering the enrichment of the target analytes on the sampler, the sensitivity of the passive sampling was better than conventional composite sampling, and it also could provide a better estimation of the infected population rather than the number of incidents. In a similar study, it has been shown that passive sampling using torpedo sampling units (containing a combination of readily available electronegative membranes, cotton buds, and medical gauze) not only makes detection of various viruses in municipal wastewater possible but also could be implemented in much smaller scales, such as an individual building, to produce point-specific data (22).
Another interesting development in the application of passive sampling in WBE is the study of illicit drugs and their metabolites and hence, the assessment of the consumption rates of such chemicals (23). Contrary to the abovementioned examples, efficient uptake of the chemicals from the complex matrix of untreated wastewater needs strong chemical sorbents and in this case a POCIS consisting of Oasis HLB (a copolymer made from hydrophilic N-vinylpyrrolidone and lipophilic divinylbenzene monomers) sandwiched between two polyether sulphone membranes was deployed. The passive sampling method was calibrated in situ for drugs such as cocaine, amphetamine, methamphetamine, and morphine. A few other biomarkers, such as the antihistamine cetirizine and metoprol acid (a metabolite of metoprolol and atenolol), which are more regularly consumed and released into wastewater, were simultaneously analyzed to produce a better estimation of the population, despite its temporal variations.
The highly variable flow of the surface water, especially rivers, could be a serious challenge since it has a dramatic effect on the sampling rate and hence the accuracy and precision of quantitative data. A helpful technique to compensate for the environmental condition changes is to use performance and reference compounds (PRCs). These compounds are selected so that they do not naturally exist in the studied water and are spiked to the sorbent prior to the deployment of the sampler. Then, the dissipation of these compounds during the sampling period is used to estimate and correct the uptake rate of the target analytes. It has been shown that this technique is suitable for hydrophobic (nonpolar) sorbents (24) but the results are not as reliable for hydrophilic samplers. As a solution, a novel approach has been introduced in which a PRC-spiked nonpolar silicon disk has been implemented in parallel with the polar sampler so that the latter was implemented for collecting and quantitating the polar target compounds (of diverse classes) and the former was used to estimate the changes in the sampling rates and to correct the quantitative results (25).
Passive sampling has also been deployed for groundwater monitoring. Regarding the significantly slower movement of the water body, the target analytes could reach an approximate equilibrium between water and the sampler sorbent, a fact that facilitates the calibration of the passive sampler. In addition, spatial (vertical) distribution of the contaminants, as an important feature of groundwater contamination studies, could be determined by deploying so-called diffusive equilibrium high-resolution passive samplers. This type of passive sampler consists of a series of sorbent cells installed inside a stainless steel rod which could be inserted into the groundwater, and the sampling process takes place at different depths. The approach has been reported to be a versatile means for studying groundwater contamination with per- and polyfluoroalkyl substances (PFAS) (26).
Study of sediments is another field in which passive samplers could play an important role. As an example, a passive sampling strategy based on POCIS has been developed for the determination of PFAS in sediments (as well as water) and the results have been used to estimate the bioaccumulation potential of these compounds (27). Beside advantages such as preconcentration of the analytes and time-weighted average concentration values, passive samplers could imitate the uptake behavior of the living organisms and therefore, provide helpful information on the impacts of the contaminants.
Passive samplers have been used in the determination of diverse air contaminants over the past five decades. There are numerous examples in this field, however, studying the transport and exchange of contaminants between different environmental media, using passive samplers, is attracting increasing focus. For example, diffusive exchange flux between the atmospheric gas phase and the freely dissolved water phase of a large set of hydrophobic organic compounds has been studied using the data extracted from passive sampling of water (LPDE and SR) and air (PUF) in high-mountain lakes (28). Such studies could reveal the transport of atmospheric contaminants and also the role of the local anthropogenic pollution sources in the contamination of remote ecosystems.
Regarding the long deployment time of passive samplers which enables collection of both long-term and temporary contamination events in the medium, as well as their preconcentration capability, the number of the collected chemical compounds and also their concentrations could be much higher compared with methods that use grab sampling. Therefore, passive sampling can be of greatest importance in non-targeted analysis, as a recent and growing field of environmental analytical chemistry (29). It has also been shown that when passive sampling is followed by sensitive analytical instrumentation, such as liquid chromatography–high resolution mass spectrometry (LC– HRMS), the number of detected compounds is increased in such a way that implementing advanced data processing methods could become inevitable (30).
Conclusion
It is clear now that despite the difficulty of calibrating passive samplers for quantitative analyses, they benefit from significant advantages such as time-weighted average data, greener analytical methods, and more time, effort, and cost-effective procedures that rationalize their implementation. That is the reason behind the spreading use of this sampling strategy, as can be seen in its progressive novel application fields. Regarding the general modality of this short review, the theoretical aspects and the entire range of the diverse application fields of passive sampling have not been covered and the interested reader is encouraged to refer to more comprehensive and specialized review articles (6).
References
(1) Godlewska, K.; Stepnowski, P.; Paszkiewicz, M. Pollutant Analysis Using Passive Samplers: Principles, Sorbents, Calibration and Applications. A Review. Environ. Chem. Lett. 2021, 19 (1), 465-520. DOI: 10.1007/s10311-020-01079-6
(2) Silvani, L.; Riccardi, C.; Eek, E.; Paini, M. P.; Morin, N. A. O.; Cornelissen, G.; Oen, A. M. P.; Hale, S. E. Monitoring Alkylphenols in Water Using the Polar Organic Chemical Integrative Sampler (POCIS): Determining Sampling Rates via the Extraction of PES Membranes and Oasis Beads. Chemosphere 2017, 184, 1362–1371. DOI: 10.1016/j.chemosphere.2017.06.083
(3) Tapie, N.; Devier, M. H.; Soulier, C.; Creusot, N.; Le Menach, K.; Aït-Aïssa, S.; Vrana, B.; Budzinski, H. Passive Samplers for Chemical Substance Monitoring and Associated Toxicity Assessment in Water. Water Sci. Technol. 2011, 63 (10), 2418–2426. DOI: 10.2166/wst.2011.129 (acccessed 8/20/2022).
(4) Thomatou, Α.-Α.; Zacharias, I.; Hela, D.; Konstantinou, I. Passive Sampling of Selected Pesticides in Aquatic Environment Using Polar Organic Chemical Integrative Samplers. Environ. Sci. Pollut. Res. 2011, 18 (7), 1222-1233. DOI: 10.1007/s11356-010-0436-6
(5) Bartkow, M. E.; Jones, K. C.; Kennedy, K. E.; Holling, N.; Hawker, D. W.; Müller, J. F. Evaluation of Performance Reference Compounds in Polyethylene-Based Passive Air Samplers. Environ. Pollut. 2006, 144 (2), 365-370. DOI: 10.1016/j.envpol.2005.12.043
(6) Salim, F.; Górecki, T. Theory and Modelling Approaches to Passive Sampling. Environ. Sci.: Processes Impacts 2019, 21 (10), 1618-1641. DOI: 10.1039/C9EM00215D
(7) Allinson, M.; Cassidy, M.; Kadokami, K.; Besley, C. H. In situ Calibration of Passive Sampling Methods for Urban Micropollutants Using Targeted Multiresidue GC and LC Screening Systems. Chemosphere 2023, 311, 136997. DOI: 10.1016/j.chemosphere.2022.136997
(8) Huckins, J. N.; Tubergen, M. W.; Manuweera, G. K. Semipermeable Membrane Devices Containing Model Lipid: A New Approach to Monitoring the Bioavaiiability of Lipophilic Contaminants and Estimating Their Bioconcentration Potential. Chemosphere 1990, 20 (5), 533-552. DOI: 10.1016/0045-6535(90)90110-F
(9) Booij, K.; Sleiderink, H. M.; Smedes, F. Calibrating the Uptake Kinetics of Semipermeable Membrane Devices Using Exposure Sandards. Environ. Toxicol. Chem. 1998, 17 (7), 1236–1245. DOI: 10.1002/etc.5620170707
(10) Lohmann, R. Critical Review of Low-Density Polyethylene’s Partitioning and Diffusion Coefficients for Trace Organic Contaminants and Implications for Its Use As a Passive Sampler. Environ. Sci. Tech. 2012, 46 (2), 606-618. DOI: 10.1021/es202702y
(11) Kingston, J. K.; Greenwood, R.; Mills, G. A.; Morrison, G. M.; Björklund Persson, L. Development of a Novel Passive Sampling System for the Time-Averaged Measurement of a Range of Organic Pollutants in Aquatic Environments. J. Environ. Monit. 2000, 2 (5), 487-495. DOI: 10.1039/B003532G
(12) Alvarez, D. A.; Stackelberg, P. E.; Petty, J. D.; Huckins, J. N.; Furlong, E. T.; Zaugg, S. D.; Meyer, M. T. Comparison of a Novel Passive Sampler to Standard Water-Column Sampling for Organic Contaminants Associated with Wastewater Effluents Entering a New Jersey Stream. Chemosphere 2005, 61 (5), 610-622. DOI: 10.1016/j.chemosphere.2005.03.023
(13) Chen, Y.; Pawliszyn, J. Kinetics and the On-Site Application of Standards in A Solid-Phase Microextration Fiber. Anal. Chem. 2004, 76 (19), 5807-5815. DOI: 10.1021/ac0495081
(14) Schuster, J. K.; Gioia, R.; Breivik, K.; Steinnes, E.; Scheringer, M.; Jones, K. C. Trends in European Background Air Reflect Reductions in Primary Emissions of PCBs and PBDEs. Environ. Sci. Tech. 2010, 44 (17), 6760-6766. DOI: 10.1021/es101009x
(15) Paschke, H.; Popp, P. New Passive Samplers for Chlorinated Semivolatile Organic Pollutants in Ambient Air. Chemosphere 2005, 58 (7), 855-863. DOI: 10.1016/j.chemosphere.2004.09.045
(16) Esteve-Turrillas, F. A.; Ly-Verdú, S.; Pastor, A.; de la Guardia, M. Development of a Versatile, Easy and Rapid Atmospheric Monitor for Benzene, Toluene, Ethylbenzene and Xylenes Determination in Air. J. Chromatogr. A 2009, 1216 (48), 8549-8556. DOI: 10.1016/j.chroma.2009.10.001
(17) Shoeib, M.; Harner, T. Characterization and Comparison of Three Passive Air Samplers for Persistent Organic Pollutants. Environ. Sci. Tech. 2002, 36 (19), 4142-4151. DOI: 10.1021/es020635t
(18) Gong, P.; Wang, X.; Liu, X.; Wania, F. Field Calibration of XAD-Based Passive Air Sampler on the Tibetan Plateau: Wind Influence and Configuration Improvement. Environ. Sci. Tech. 2017, 51 (10), 5642-5649. DOI: 10.1021/acs.est.7b01029
(19) Oono, S.; Harada, K. H.; Mahmoud, M. A. M.; Inoue, K.; Koizumi, A. Current Levels of Airborne Polyfluorinated Telomers in Japan. Chemosphere 2008, 73 (6), 932-937. DOI: 10.1016/j.chemosphere.2008.06.069
(20) Sims, N.; Kasprzyk-Hordern, B. Future Perspectives of Wastewater-Based Epidemiology: Monitoring Infectious Disease Spread and Resistance to the Community Level. Environ. Int. 2020, 139, 105689. DOI: 10.1016/j.envint.2020.105689
(21) Breulmann, M.; Kallies, R.; Bernhard, K.; Gasch, A.; Müller, R. A.; Harms, H.; Chatzinotas, A.; van Afferden, M. A Long-Term Passive Sampling Approach for Wastewater-Based Monitoring of SARS-CoV-2 in Leipzig, Germany. Sci. Total Environ. 2023, 887, 164143. DOI: 10.1016/j.scitotenv.2023.164143
(22) Mejías-Molina, C.; Pico-Tomàs, A.; Beltran-Rubinat, A.; Martínez-Puchol, S.; Corominas, L.; Rusiñol, M.; Bofill-Mas, S. Effectiveness of Passive Sampling for the Detection and Genetic Characterization of Human Viruses in Wastewater. Environ. Sci.: Water Res. Technol. 2023, 9 (4), 1195-1204, 10.1039/D2EW00867J. DOI: 10.1039/D2EW00867J
(23) Harman, C.; Reid, M.; Thomas, K. V. In Situ Calibration of a Passive Sampling Device for Selected Illicit Drugs and Their Metabolites in Wastewater, And Subsequent Year-Long Assessment of Community Drug Usage. Environ. Sci. Tech. 2011, 45 (13), 5676-5682. DOI: 10.1021/es201124j
(24) Booij, K.; Smedes, F. An Improved Method for Estimating in Situ Sampling Rates of Nonpolar Passive Samplers. Environ. Sci. Tech. 2010, 44 (17), 6789-6794. DOI: 10.1021/es101321v
(25) Glanzmann, V.; Reymond, N.; Weyermann, C.; Estoppey, N. An Improved Chemcatcher-Based Method for the Integrative Passive Sampling of 44 Hydrophilic Micropollutants in Surface Water – Part A: Calibration Under Four Controlled Hydrodynamic Conditions. Sci. Total Environ. 2023, 871, 162037. DOI: 10.1016/j.scitotenv.2023.162037
(26) McDermett, K. S.; Guelfo, J.; Anderson, T. A.; Reible, D.; Jackson, A. W. The Development of Diffusive Equilibrium, High-Resolution Passive Samplers to Measure Perfluoroalkyl Substances (PFAS) in Groundwater. Chemosphere 2022, 303, 134686. DOI: 10.1016/j.chemosphere.2022.134686
(27) Atoufi, H. D.; Lampert, D. J. Analysis of a Passive Sampling Device to Assess the Behavior of Per- and Polyfluoroalkyl Substances in Sediments. Environ. Toxicol. Chem. 2023, 42 (10), 2171-2183. DOI: https://doi.org/10.1002/etc.5705
(28) Prats, R. M.; van Drooge, B. L.; Fernández, P.; Grimalt, J. O. Passive Water Sampling and Air–Water Diffusive Exchange of Long-Range Transported Semi-Volatile Organic Pollutants in High-Mountain Lakes. Sci. Total Environ. 2023, 860, 160509. DOI: 10.1016/j.scitotenv.2022.160509
(29) Wang, S.; Basijokaite, R.; Murphy, B. L.; Kelleher, C. A.; Zeng, T. Combining Passive Sampling with Suspect and Nontarget Screening to Characterize Organic Micropollutants in Streams Draining Mixed-Use Watersheds. Environ. Sci. Tech. 2022, 56 (23), 16726-16736. DOI: 10.1021/acs.est.2c02938
(30) Hohrenk-Danzouma, L. L.; Vosough, M.; Merkus, V. I.; Drees, F.; Schmidt, T. C. Non-Target Analysis and Chemometric Evaluation of a Passive Sampler Monitoring of Small Streams. Environ. Sci. Tech. 2022, 56 (9), 5466-5477. DOI: 10.1021/acs.est.1c08014
Amir Salemi and Torsten C. Schmidt are with the Faculty of Chemistry at the University of Duisburg-Essen, in Essen, Germany. Schmidt is also with the Centre for Water and Environmental Research at the University of Duisburg-Essen, in Essen, Germany, and IWW Water Centre in Mülheim an der Ruhr, Germany. Direct correspondence to: amir.salemi@gast.uni-due.de

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.