Onsite Environmental Extraction Based on Portable and Affordable Stirred Devices
Environmental compartments are characterized by their large size and the heterogeneous distribution of the target analytes. Onsite extraction procedures are especially useful in this scenario, allowing the development of ambitious sampling campaigns (including a larger number of locations and periods). This article outlines the relevance of extraction techniques, including exhaustive and non-exhaustive ones, in onsite strategies. However, only stirred units are discussed and described in detail. The discussion of the analytical performance (for example, sensitivity and precision) is intentionally avoided to focus the attention on the devices that can be applied (selecting the sorptive phase) to almost any analytical problem. The impact of open technologies (microprocessors and 3D printing) in the design of these units is also presented.
Onsite environmental analysis is a simplified procedure that measures the target analytes at the sampling point. This strategy reduces errors by eliminating unnecessary steps that add to the total variance of the results (1). Onsite analysis also maintains the sample’s representativeness by avoiding storage and transportation, which can alter the sample composition (in ways such as, but not limited to, analyte losses by adsorption in the vessel walls). However, onsite analysis often depends on portable instruments with lower performance than benchtop facilities. The sensitivity and selectivity can be improved by applying an onsite sample preparation step. This combined approach is practical for those problems where the sample matrices are not very complex, and the concentration thresholds are not very strict. If these conditions are not fulfilled, benchtop instruments cannot be avoided. In this case, onsite extraction can be developed in a different way. The analytes are in situ isolated and retained in a sorptive phase, which can be liquid or solid. Once retained, the analytes are less prone to be lost by evaporation or chemical degradation (if the phase is appropriately dried and stored). The sorptive phase is transported to the laboratory, where it can be stored for the final analysis. The portability and affordability of the sample preparation devices are essential in making the last approach practical and useful.
A sample preparation procedure should be as simple and miniaturized as possible to be portable. A closer look at these characteristics reveals some sustainability-related connotations. In fact, simplicity and miniaturization result in procedures with a lower requirement of consumables (solvents, reagents) and energy, thus reducing the environmental impact. It is not surprising that López-Lorente and associates selected portability as the first of the ten principles of green sample preparation (GSP) (2).
The distribution of target analytes in a given environmental compartment is non-uniform, varying both spatially and temporally. Therefore, to have a complete understanding of this distribution, it is necessary to take multiple samples from different locations and at different times. The cost of this multisampling approach dramatically depends on the price of the extraction units. Therefore, affordable devices permit the definition of more ambitious sampling campaigns.
Thanks to their inherent characteristics, microextraction techniques are ideal tools for onsite sample preparation. Solid-phase microextraction (SPME) and related techniques are paradigmatic in this sense. Their miniaturized nature also allows the design of special sampling devices capable of extracting the analytes at difficult-to-access locations. For example, Grandy and co-authors integrated a thin film microextraction device in a floating aerial drone for onsite water extraction at remote locations (3). In the same way, SPME devices have been implemented in aerial drones for environmental air sampling and extraction (4). Readers interested in deepening onsite environmental sample preparation can find further information in recent literature where the topic has been reviewed (5,6). Instead, this article focuses on our experience developing onsite environmental extraction based on stirred devices.
Exhaustive and Non-Exhaustive Extraction Techniques
Green analytical chemistry (GAC) (7) and GSP (2) principles recommend minimizing the sample volume, as this parameter directly influences the resources needed for the analytical procedure. Environmental analysis is particular since the large size of the studied compartments requires a relatively high sample volume to assess its representativeness. Onsite processing of these volumes (typically in the range of hundreds of mL to a few of L) is challenging since close contact of the sample with the sorptive phase is required to isolate the analytes efficiently. Additionally, the sample volume defines the amount of analyte that is extracted. This effect, which influences the sensitivity of the determination, is typically more marked in exhaustive extractions where the sample volume must be precisely measured. The need to accurately measure the sample volume when applying a non-exhaustive technique depends on the experimental conditions under which the extraction is carried out. In fact, in SPME, when the sample is much larger than the sorptive phase volume, the amount of analyte extracted becomes independent of the sample volume. Rather than being a drawback, this fact allows the development of in situ extraction, where the sorptive phase is directly introduced in the environmental compartment without an apparent (although it is implicit) sampling step. The effect of the sample volume must be understood before designing an onsite extraction.
In solid-phase extraction (SPE), the sample is forced to flow through the sorbent bed, thus increasing the efficiency. In this case, Schulze and associates reported a device capable of onsite processing up to 50 L of sample (8). The device consisted of a customizable SPE cartridge that can be filled with the adequate sorbent, or mixture of sorbents, ad-hoc selected depending on the target analytes. A prefiltration element is incorporated to avoid clogging the cartridge with the suspended particulate matter. A pressurized system is implemented to flow the sample through the cartridge. The device has a power demand of 12 V that can be supplied using a car battery.
In nonexhaustive extraction approaches, like SPME, the extraction significantly depends on the diffusion of the analytes from the bulk sample to the sorptive phase. The sample agitation typically improves this diffusion. In 2018, Piri-Moghadam and associates described a bottle-based apparatus for onsite extraction (9). In this approach, 1 L of the sample is initially taken in a glass bottle and closed with a polytetrafluoroethylene (PTFE) stopper. The thin film microextraction (TFME) phase hangs from the stopper, held by a polymeric thread. A sinker, placed at the end of the thread, guarantees the immersion of the sorptive phase into the sample. An orbital agitator is used to enhance the diffusion of the analytes. Liu and co-authors recently reported an onsite extraction technique consisting of a glass bottle whose inner surface were coated with a polydimethylsiloxane/divinylbenzene film (10). The sample is introduced into the bottle and stirred to retain the analytes (volatile hydrocarbons in this particular example) in the coated walls of the bottle. After the extraction, the sample is discharged, and the vial can be transported to the lab or analyzed in a portable chromatograph.
Onsite Extraction Using Stirred Devices
Drills are cheaper and more portable than orbital shakers and magnetic stirrers and can be operated with batteries. Moreover, they open the possibility of integrating the sorbent phase into the stirring element, which is an additional advantage for the diffusion of the analytes. In 2009, Qin and coauthors proposed using planar sorptive phases coupled to a portable drill as a miniaturized extraction system in environmental analysis (11). Mao and associates have also proposed the combination of stir-bar sorptive extraction (SBSE) with a portable electric drill (12). In this case, the stir-bar is magnetically attached to the drill that is used for sample agitation.
To improve the versatility of the sorptive phases available, our research group described the synthesis of borosilicate disks modified with carbon nanohorns as a sorbent phase for the extraction of an endocrine disruptor, benzophenone-3, from water samples (13). As shown in Figure 1, the disks were easily attached to a portable drill that allowed them to be agitated inside the sample. This agitation improved the diffusion of the analytes, allowing their quick isolation, thus facilitating sampling at different locations in a single working day.
FIGURE 1: (a) Raw borosilicate disk (1) and disk modified with o-SWNHs (oxidized single-wall nanohorns) (2); (b) Attachment of the disk to a metallic axle; (c) Assembly of the disk to the drill; (d) The extraction process. Reproduced with permission of Elsevier from reference (13).

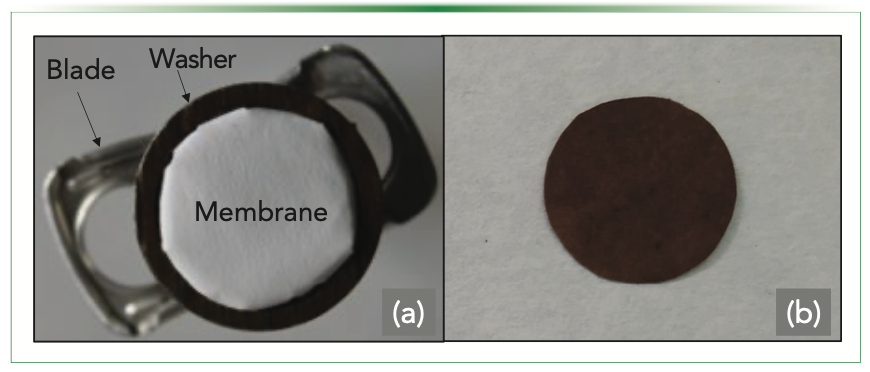
Carbon disks provided outstanding performance. However, they were not commercially available, thus restricting their potential application in routine analysis. To overcome this limitation, commercial polymeric membranes were proposed as sorbent phases in 2019 (14). The wide diversity of commercial membranes significantly increased the technique’s applicability for extracting different contaminants. The robust coupling of these membranes with portable drills posed a challenge. Magnets were proposed as extraction supports to achieve this integration. The polymeric membranes were placed on top of the magnet to which they were attached using a metal washer. Blades were attached to the unit to improve the stirring capability of the devices. The extraction unit is represented in Figure 2a. Although the initial results were promising, the metal washer seemed to reduce the extraction kinetics by creating a reduced diffusion layer over the sorptive phase. To solve the latter limitation, magnetic membranes were proposed as sorptive phases in 2021 (15). The magnetic membranes (Figure 2b), prepared by the control coating of a paper with a polymeric magnetic nanocomposite, could be attached to the magnet directly. Avoiding the metallic washer permits the direct interaction of the sorptive phase with the sample.
FIGURE 2: (a) Extraction unit designed for polymeric membranes. The membranes are attached to the magnet by a metallic washer; (b) Magnetic membrane synthesized by coating a paper circle into a precursor solution containing a polymeric nanocomposite. Panel (b) reproduced with permission of Elsevier from reference (15).
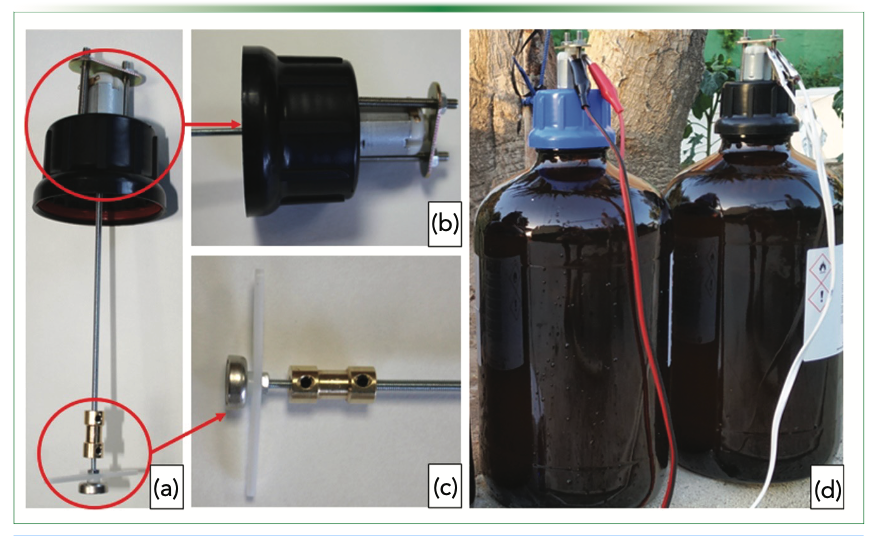
Making Stirred Devices Affordable
Simplicity is an added value in onsite extraction techniques as it directly impacts the price of the designed units. In the previous investigations, portable drills were used to agitate the sorbent phases. The price of these drills, around 50 € ($53.00), and the need to recharge their batteries relatively frequently are significant handicaps in designing ambitious sampling campaigns. In 2022, we evaluated the replacement of these drills with electric mini-motors (16). The reduced cost of these motors (about 0.4 € [$0.42] per unit), opens the possibility of manufacturing many sampling devices, thus increasing the number of locations that can be sampled. In addition, these motors operate efficiently with portable 5V batteries, giving them greater autonomy. Figure 3a shows the designed extraction unit. The electric motor is integrated into the stopper of a glass bottle (Figure 3b). The extraction element (a magnet on which the sorbent phase is fixed) is located at the bottom of the unit, where a blade is also placed to improve the diffusion of the analytes (Figure 3c). Both elements, motor and magnet, are connected by a metal rod that transmits the movement. The extraction procedure involves several well-defined sequential steps. Initially, a sample volume in the range of 2.5 L is taken and placed in the glass bottle (Figure 3d). The extraction unit is introduced into the sample by simply closing the stopper, and then 5 V is applied to the motor to agitate the sorptive phase inside the sample. After the extraction, the sorptive phase is recovered and transported to the laboratory for final analysis. A new sorptive phase was designed to boost the extraction of the target analytes. Hydrophilic lipophilic balance particles, which are a common sorbent in environmental analysis, were immobilized using adhesive tape as the binder in a magnetic tape. The microparticles were fixed on the surface of the tape, having a high superficial area to contact with the analytes.
FIGURE 3: (a) Extraction device; (b) Electric motor attached to the plastic stopper; (c) Magnet holder and blades for better agitation; (d) Onsite extraction. Reproduced with permission of Elsevier from reference (16).
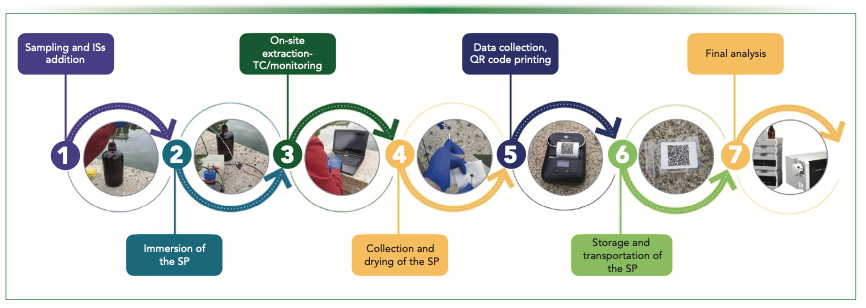
Open technologies can play a fundamental role in lowering the fabrication costs of these devices. Recently, the research group has developed a device based on Arduino technology that also integrates sensors for online monitoring of various parameters (17). A temperature sensor and conductivity sensor are incorporated into the stopper, allowing the measurement of these parameters during the onsite extraction. The motor is operated by the Arduino microprocessor that controls the extraction time. The onsite extraction procedure is schematically presented in Figure 4, consisting of well-defined steps. Initially, a defined sample volume is taken, and the internal standard is added (Figure 4.1). The extraction is performed while the temperature and conductivity values are monitored (Figure 4.2–3). After the extraction, the sorptive phase is dried (Figure 4.4), and a QR code label containing the information of the sample (GPS coordinates of the sampling point, temperature, conductivity, and other data) is printed in a portable printer (Figure 4.5). The QR label is attached to a plastic bag (Figure 4.6) where the sorptive phase is stored for its transportation to the lab for final analysis (Figure 4.7).
FIGURE 4: Analytical procedure for the Arduino-controlled onsite extraction device. For details, see text. Reproduced with permission of Elsevier from reference (17).
3D printing is a disruptive technology with broad applications in analytical chemistry, and it has been recently used to fabricate stirred extraction devices to be deployed onsite (18). Vargas-Muñoz and associates designed a stirrer whose paddles were 3D printed and coated with a metal-organic framework to extract phenols. After the extraction, the paddles can be removed from the stirrer and chemically eluted before the analysis.
Conclusion
Onsite sample preparation is a useful strategy in environmental analysis with positive effects on the metrological quality (representativeness) and the affordability (easier sampling logistics) of the analytical procedures. Extraction techniques, both exhaustive and non-exhaustive ones, have demonstrated potential in this field. However, due to their miniaturized and simplified character, microextraction technique seems to be a better option. This article outlined the role that stirred-based techniques can play in onsite extraction, presenting our perspective on the topic. The introduction of open technologies (microprocessors and 3D printing) in the design of these devices improves their affordability, which is critical to extending their use. There is room for improvement in this field. Directly coupling these onsite devices with instrumental techniques (spectroscopic and spectrometric ones) can simplify the analytical procedures even more.
Acknowledgments
Financial support from the Andalusian counseling of “Transformación Económica, Industria, Conocimiento y Universidades” (PY20_00461) is gratefully acknowledged. This article is based upon work from the Sample Preparation Study group and Network, supported by the Division of Analytical Chemistry of the European Chemical Society.
References
(1) Pawliszyn, J. Why Move Analysis from Laboratory to On-Site? TrAC, Trends Anal. Chem. 2006, 25 (7), 633–634. DOI: 10.1016/j.trac.2006.06.002
(2) López-Lorente Á. I. et al. The Ten Principles of Green Sample Preparation. TrAC, Trends Anal. Chem. 2022, 148, 116530. DOI: 10.1016/j.trac.2022.116530
(3) Grandy, J. J.; Galpin, V.; Singh, V.; Pawliszyn, J. Development of a Drone-Based Thin-Film Solid-Phase Microextraction Water Sampler to Facilitate On-Site Screening of Environmental Pollutants. Anal. Chem. 2020, 92(19), 12917–12924. DOI: 10.1021/acs.analchem.0c01490
(4) Ruiz-Jimenez, J. et al. Aerial Drone as a Carrier for Miniaturized Air Sampling Systems. J. Chromatogr. A 2019, 1597, 202–208. DOI: 10.1016/j.chroma.2019.04.009
(5) Sajid, M. Advances in On-Site Analytical Sample Preparation for Analysis of Environmental Waters: A Review. Trends in Environ. Anal. Chem. 2022, 36, e00175. DOI: /10.1016/j.teac.2022.e00175
(6) Casado-Carmona, F. A.; Lasarte-Aragonés, G.; Lucena, R.; Cárdenas, S. "Green Sample Preparation Techniques in Environmental Analysis" in Green Approaches for Chemical Analysis; Elsevier, 2023. pp. 241-276.
(7) Gałuszka, A.; Migaszewski, Z.; Namieśnik, J.The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC, Trends Anal. Chem. 2013, 50, 78–84. DOI: 10.1016/j.trac.2013.04.010
(8) Schulze, T. et al. Assessment of a Novel Device for Onsite Integrative Large-Volume Solid Phase Extraction of Water Samples to Enable a Comprehensive Chemical and Effect-Based Analysis. Sci. Total Environ. 2017, 581–582, 350–358. DOI: 10.1016/j.scitotenv.2016.12.140
(9) Piri-Moghadam, H.; Gionfriddo, E.; Grandy, J. J.; Alam, Md. N.; Pawliszyn, J. Development and Validation of Eco-Friendly Strategies Based on Thin Film Microextraction for Water Analysis. J. Chromatogr. A 2018, 1579, 20–30. DOI: /10.1016/j.chroma.2018.10.026
(10) Liu, X. et al. Sample Bottle Coated with Sorbent as a Novel Solid-Phase Extraction Device for Rapid On-Site Detection of BTEX in Water. Anal. Chim. Acta2021, 1152, 338226. DOI: 10.1016/j.aca.2021.338226
(11) Qin, Z.; Bragg, L.; Ouyang, G.; Niri, V. H.; Pawliszyn, J. Solid-Phase Microextraction Under Controlled Agitation Conditions for Rapid On-Site Sampling of Organic Pollutants in Water. J. Chromatogr. A 2009, 1216 (42), 6979–6985. DOI: 10.1016/j.chroma.2009.08.052
(12) Mao, X.; Hu, B.; He, M.; Fan, W. Stir Bar Sorptive Extraction Approaches with a Home-Made Portable Electric Stirrer for the Analysis of Polycyclic Aromatic Hydrocarbon Compounds in Environmental Water. J. Chromatogr. A2012, 1260, 16–24. DOI: 10.1016/j.chroma.2012.08.062
(13) Roldán-Pijuán, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Micro-Solid Phase Extraction Based on Oxidized Single-Walled Carbon Nanohorns Immobilized on a Stir Borosilicate Disk: Application to the Preconcentration of the Endocrine Disruptor Benzophenone-3. Microchem. J. 2014, 115, 87–94. DOI: 10.1016/j.microc.2014.02.015
(14) Casado-Carmona, F. A.; del C. Alcudia-León,M.; Lucena, R.; Cárdenas, S. Portable Stir Membrane Device for On-Site Environmental Sampling and Extraction. J. Chromatogr. A2019,1606, 360359. DOI: 10.1016/j.chroma.2019.07.013
(15) Casado-Carmona, F. A.; Lucena, R.; Cárdenas, S. Magnetic Paper-Based Sorptive Phase for Enhanced Mass Transference in Stir Membrane Environmental Samplers. Talanta 2021, 228,122217. DOI: 10.1016/j.talanta.2021.122217
(16) Casado-Carmona, F. A.; Jiménez-Soto, J. M.; Lucena, R.; Cárdenas, S. Portable Stirring Device for the On-Site Extraction of Environmental Waters Using Magnetic Hydrophilic-Lipophilic Balance Tape. Anal. Chim. Acta 2022, 1189, 339186. DOI: 10.1016/j.aca.2021.339186
(17) Casado-Carmona, F. A.; Lucena, R.; Cárdenas, S. Lab in a Bottle, Open-Source Technologies for the Design of Affordable Environmental Samplers Integrating On-Site Extraction, J. Environ. Chem. Eng. 2023, 11 (3), 109713, DOI: 10.1016/j.jece.2023.10971318.
(18) Vargas-Muñoz, M. A.; Palomino, C.; Turnes, G.; Palacio, E. A Sampling Platform Incorporating 3D-Printed Paddles-Stirrers Coated with Metal-Organic Framework MIL-100(Fe) for in-situ Extraction of Phenolic Pollutants in Biodigester Supernatant and Wastewater Effluent Samples. J. Environ. Chem. Eng. 2023, 11 (5), 110503. DOI: 10.1016/j.jece.2023.110503
Francisco Antonio Casado-Carmona, Rafael Lucena, and Soledad Cárdenas are with the Chemical Institute for Energy and Environment (IQUEMA), part of the Department of Analytical Chemistry, at the University of Córdoba in Córdoba, Spain. Direct correspondence to: rafael.lucena@uco.es

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)