Rapid LC Determination of UV Filters in Cosmetics using Ethanol as the Mobile Phase
LCGC Europe
A rapid and environmentally friendly LC method for the simultaneous determination of 12 UV filters in cosmetic samples using ethanol as the mobile phase.
An environmentally friendly and rapid liquid chromatography (LC) method for the simultaneous determination of 12 UV filters in cosmetic samples is described using ethanol as the mobile phase. The proposed method is validated for the analysis of 21 homemade samples and 8 commercial samples.
Solar ultraviolet (UV) radiation can cause a wide variety of adverse health reactions, such as erythema, immunosuppression, melanogenesis, photodermatoses, photoaging, mutagenic lesions and skin cancer.1 To prevent and reduce these harmful effects, the dermatologic community recommends the daily use of sunscreens.2
Active ingredients called UV filters are included in the sunscreens' formulation to provide broad-spectrum UV protection.3–5 UV filters absorb (chemical filters) or reflect (physical filters) UV radiation. The effectiveness of sunscreens is indicated by the sun protection factor (SPF) printed on the cosmetic product label and depends on the UV filters used and their concentration level. The compounds used as UV filters and their maximum allowed concentration level are regulated by European legislation.6 However, there are no official analytical methods of control.

KEY POINTS
In the bibliography there is information concerning dermatological side effects (allergy and contact dermatitis) caused by the use of sunscreens.7–9 Therefore, new analytical methods need to be developed to ensure effectiveness, safety and compliance with the law.
The analytical methods published have been examined in an interesting chapter published by Chisvert and Salvador.10 Most of these methods are based on the use of liquid chromatography (LC) and involve the use of toxic solvents such as methanol,11 tetrahydrofuran,12 acetonitrile13 or dimethylformamide.14
Since then, two more papers on this subject have been published: one discussing the use of LC with acetonitrile and tetrahydrofuran gradient15 and another discussing the use of methanol.16
According to safety pictograms, ethanol is regarded as a flammable solvent, by contrast, methanol is also toxic, tetrahydrofuran is irritating and acetonitrile and dimethylformamide are harmful. Therefore, the use of ethanol is preferable.
There are very few attractive analytical procedures that avoid the use of toxic organic solvents to determine UV filters in cosmetic samples using ethanol as a non-toxic solvent.17 One was proposed by Schake in 2004,18 which is based on LC and its run time is 32 min. The others were developed by Salvador's group and were based on flow injection spectrometric techniques,19,29 or liquid chromatography.21–24 While the methods described in the first articles19–23 can only determine a small number of UV filters, the method proposed in the last article24 allows 18 UV filters to be determined by LC using two different gradient elution programmes depending on whether water-soluble or fat-soluble UV filters are present. The run time using the gradient for fat-soluble analytes is 40 min and for water soluble is 15 min. However, three of them are derived from aminobenzoic acid (p-aminobenzoic acid, ethylhexyl dimethyl PABA and PEG-25 PABA), which are now in disuse (p-aminobenzoic acid will soon be prohibited by the European Commission), and two of them are protected by patents which restrict their use (drometrizole trisiloxane and terephthalylidene dicamphor sulphonic acid).
The aim of this work is to offer a fast analytical method that will allow 11 of the most commonly used fat-soluble UV filters (see the section Reagents and samples) to be determined, combined with the most widely-used water-soluble UV filter [phenylbenzimidazole sulphonic acid (HYDRO)] in just one run, using ethanol and in a run time of 5.5 minutes. In addition to this, this method will allow the determination of two UV filters not included in the environmentally friendly methods described in the bibliography: methylene bis-benzotriazolyl tetramethylbutylphenol (TS) and bis-ethylhexyloxyphenol methoxyphenyl triazine (TM). Only one method for TM14 and another for TS15 are described using toxic solvents. The development of new analytical methods for the determination of TM and TS is extremely important as these are organo-mineral UV filters, being used increasingly by the cosmetic community.
The use of this method permits exhaustive quality control (QC) of production in much less time than usual, with increased safety for the operator, as well as being more environmently friendly.
Experimental
Apparatus: A Hitachi Elite LaChrom (Tokyo, Japan) LC system equipped with an L-2455 diode array detector, an L-2300 column oven, an L-2200 autosampler and an L-2130 gradient pump was employed, using a Chromolith Performance RP-18e (10 cm × 4.6 mm i.d., 2 μm macropore size and 13 nm mesopore size) column from Merck (Darmstadt, Germany). An ultrasonic water bath from Branson (Danbury, USA) model 5510 was used to perform the sample solubilization.
Reagents and samples: Analytical reagent-grade acetic acid from Probus (Badalona, Spain), ethanol gradient grade and water for chromatography from Merck (Darmstadt, Germany) were used as solvents.
The standards used for the determination of UV filters were: phenylbenzimidazole sulphonic acid (HYDRO) 99%, ethylhexyl salicylate (OS) 99.8%, isoamyl methoxycinnamate (IMC) 99.5%, octocrylene (OCR) 99.0%, ethylhexyl methoxycinnamate (OMC) 99.22%, butyl methoxydibenzoylmethane (BDM) 98.9% and homosalate (HMS) 99.8% from Symrise (Holzminden, Germany); diethylhexyl butamido triazone (HEB) 97.46% from 3V Sigma (Mozzo, Italy); benzophenone-3 (BZ3) >99% and 4-methylbenzylidene camphor (MBC) >99% from Merck (Darmstadt, Germany); methylene bis-benzotriazolyl tetramethylbutylphenol (TM) 57.5% and bis-ethylhexyloxyphenol methoxyphenyl triazine (TS) 99% from Ciba (Basel, Switzerland).
Eight commercial samples containing different mixtures of the target compounds were analysed. The samples were: 1 = sunscreen cream-gel; 2 = sunscreen fluid emulsion-1; 3 = anti-ageing hand cream; 4 = sunscreen fluid emulsion-2; 5 = anti-ageing hand cream; 6 = lip protector stick; 7 = facial sunscreen cream; and 8 = de-pigmentation cream.
Twenty-one samples containing many combinations of these 12 UV filters were produced in accordance with the usual procedures followed by the cosmetic industry. The standard samples were prepared following the actual protocols (laboratory scale) used by "Laboratorios Babé" and "RNB Cosméticos". Therefore, these cosmetic products are equivalent to those found in supermarkets and pharmacies. The formulations of these products are protected by professional secrecy. In general, the fatty components and aqueous components are weighed separately in two beakers of 500 mL. The beakers are heated to about 75 °C. After, the fatty phase is slowly added on to the aqueous phase to produce an emulsion with constant agitation (UltraTurrax). Finally, the emulsion is cooled below 40 °C and the last thermolabile components (preservatives, perfumes) were added. Klein and Palefsky have published representative formulations of sunscreen products.25
The samples were: 1 = after-shave; 2 = moisturiser cream-gel; 3 = facial cream-gel; 4 = sunscreen lotion-1; 5 = anti-wrinkle cream; 6 = moisturiser cream; 7 = eau de toilette; 8 = anti-ageing cream; 9 = sunscreen lotion-2; 10 = sunscreen fluid emulsion-1; 11 = sunscreen fluid emulsion-2; 12 = sunscreen milk; 13 = sunscreen milk-2; 14 = facial cream-1; 15 = anti-ageing sunscreen; 16 = anti-ageing hand cream; 17 = sunscreen lotion-3; 18 = facial cream-2; 19 = facial cream-3; 20 = sunscreen lotion-3; and 21 = lip protector stick.
These samples were analysed and the results compared with known theoretical values in order to test their accuracy.
Proposed method: A 1000 μg/mL multi-component stock solution of the 12 UV filters was prepared in ethanol (containing 1 drop of 10% sodium hydroxide to dissolve HYDRO). The working standard solutions were prepared taking aliquots from the stock solution (from 20–100 μg/mL).
0.01–0.05 g of samples were weighed into a 10 mL volumetric flask, diluted with ethanol and dissolved using an ultrasonic bath for 15 min. Finally, the sample solution was filtered through a 0.45 μm nylon membrane filter. Standard or sample solutions were injected (5 μL) into the chromatographic system. The column temperature was set at 30 °C, the flow rate was 3 mL/min and detection was performed at 306 nm (BDM was measured at 360 nm to increase its sensibility and accuracy.24
The separation of UV filters was performed using the gradient elution shown in Table 1.
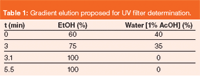
Table 1: Gradient elution proposed for UV filter determination.
Results and discussion
Sample preparation: The solvent used to prepare samples and the time for sonication, were optimized. Different ethanol and water proportions were studied, and finally 100% ethanol was chosen because it breaks up the sample matrix best and has good solubility for the targeted UV filters. Total extraction of UV filters in samples was performed after 15 min of sonication.
Study of the chromatographic separation: Initially, the gradient elution used was ethanol: water (1% acetic acid) 70:30 (v/v) to 100% ethanol in 10 min and the flow rate was 1.5 mL/min (column temperature was 45 °C). To reduce the run time, flow rates of 1.5, 2.0, 2.5, 3.0 and 3.5 mL/min were assayed. The flow rate of 3 mL/min was selected to show lower retention times (less than 5 min), higher flow rates were discarded to increase the maximum operating pressure for the column.
However, the peaks for the IMC–MBC and OMC–BDM pairs overlapped respectively. A significant improvement was achieved using an initial 60% ethanol (to 100% ethanol in 10 min) and a temperature of 30 °C. The peak separation of OMC and BDM was solved, but IMC and MBC were still overlapped. To solve this problem, different gradient steps were studied. Finally, separation of the 12 UV filters was performed using the gradient steps shown in Table 1.
A representative chromatogram using these optimized conditions is shown in Figure 1.
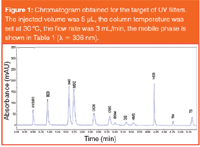
Figure 1
Statistical information: The repeatability of the measurements was tested by injecting a multi-component standard solution (around 50 μg/mL of each UV filter) five times. The retention time (obtained by five parallel measurements) of UV filters is 0.495±0.02 min [relative standard deviation (RSD) was 4%] for HYDRO, 0.876±0.006 min (RSD was 0.7%) for BZ3, 1.494±0.009 min (RSD was 0.6%) for IMC, 1.650±0.009 min (RSD was 0.5%) for MBC, 2.266±0.009 min (RSD was 0.4%) for OCR, 2.765±0.007 min (RSD was 0.3%) for OMC, 2.940±0.007 min (RSD was 0.2%) for BDM, 3.283±0.006 min for OS (RSD was 0.2%), 3.501±0.006 min (RSD was 0.2%) for HMS, 4.190±0.003 min (RSD was 0.1%) for HEB, 4.763±0.009 min (RSD was 0.2%) for TM and 5.343±0.008 min (RSD was 0.1%) for TS. The RSD in peak areas was between 0.5% and 1.5%. The calibration parameters and linearity of each UV filter are shown in Table 2.
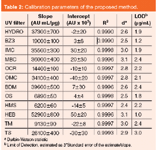
Table 2: Calibration parameters of the proposed method.
The linearity was validated according to the regression coefficients (R2) and the Durbin-Watson statistic (d) was used to detect the absence of autocorrelation in the residuals. The regression coefficients and the Durbin-Watson statistic were greater than 0.99 and 2 respectively in all cases, validating the linear regression models. The limits of detection are low (between 1–3 μg/mL) and allow the analysis of all the samples studied. The sensitivity of the proposed method for each UV filter is defined as the slope of the calibration graph (shown in Table 2).26
The inter-day RSD for each UV filter was calculated based on the slope obtained on five different days. The inter-day RSD was between 2.3% and 4.6%. The mean value was 3.6%. To verify the accuracy of the proposed method,21 homemade samples (described in the section Reagents and samples) were analysed and the results were compared with the known theoretical values. These results are shown in Table 3.
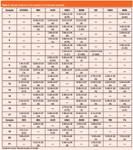
Table 3: Results obtained in the analysis of homemade samples.
The results obtained by the proposed method were statistically compared with the theoretical values using a linear regression model. The results obtained were represented on the ordinate axis (Y) and the theoretical values on the abscise axis (X). The equation obtained was: Y = (0.999±0.003)X + (0.02±0.02), R2 = 0.9990, N = 90.
The confidence interval (5% significance level and N–2 degrees of freedom) for the slope are 0.993 to 1.006, and for the intercept are –0.01 to 0.05. Therefore, intervals include values 1 and 0 respectively. These results demonstrate that the proposed method provides statistically accurate results. Finally, the proposed method was applied to eight commercial samples (described in the section Reagents and samples) the results of which are shown in Table 4.
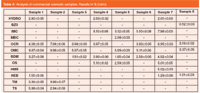
Table 4: Analysis of commercial cosmetic samples. Results in % (m/m).
Representative chromatograms of all commercial samples are shown in Figure 2. Peak purity control was performed to verify that the peaks did not co-elute with small impurities; the purity level for peaks was between 98% and 100%.
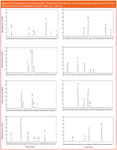
Figure 2
Conclusions
We have developed a rapid, environmentally friendly liquid chromatography method for the simultaneous determination of 12 UV filters in cosmetic samples.To our knowledge, no other published method allows methylene bis-benzotriazolyl tetramethylbutylphenol (TM) and bis-ethylhexyloxyphenol methoxyphenyl triazine (TS) determination using non-toxic solvents.
The proposed method is accurate, precise and sensitive and is suitable for the analysis of different types of cosmetics (emulsions oil/water, water/oil, lotions, lipsticks, etc.). The run time is just 5.5 min. At every step, the proposed method employs non-toxic solvents only and allows rapid and exhaustive quality control of production. It is safe for the operator and environmently friendly, meeting the needs of the cosmetics industry.
Ethanol is not commonly used as a mobile phase compared with traditional solvents, such as methanol or acetonitrile, which are more toxic. However, some laboratories are beginning to use ethanol more as part of a green chemistry initiative. The organic solvent can be easily changed to ethanol when the chromatographic peaks using traditional solvents are well resolved and the working pressure is not high. Ethanol may, however, cause peak broadening and the need to work at much higher pressures.
Acknowledgements
The authors wish to thank Dr A. Salvador, Dr A Chisvert and their research group in the Analytical Chemistry Department of the University of Valencia for their analytical technical assistance.
Ángel Balaguer obtained his doctorate in analytical chemistry in September 2008. The research for this qualification was based on the development of analytical methods for estimating the safety and quality control of cosmetic products. He currently works at "RNB Cosméticos" and has been manager of the analytical department (R&D) since January 2008.
Silvia Talamantes gained her degree in chemistry in 2006. She currently works at "RNB Cosméticos" and has been a technical specialist in the analytical department (R&D) since November 2005.
Eva de les Neus Duran Giner obtained her degree in chemistry in 2008. She currently works at "RNB Cosméticos" and has been a technical specialist in the analytical department (R&D) since May 2006. She is pursuing a Masters of experimental and industrial organic chemistry.
Pascual Cuadrado Escamilla obtained his degree in chemistry in 1993 and his Masters of cosmetology and dermopharmacie in 1995. He has been a member of the Spanish Society of Cosmetic Chemists since 1996. He pursued doctorate courses during 2001–2002 in the Department of Analytical Chemistry (University of Valencia). He has been the director of the R&D Department at "RNB Cosméticos" since 1997.
References
1. L. Marrot and J.R. Meunier, J. Am. Academy of Dermatology, 58, S139–S148 (2008).
2. T.J. Phillips et al., J. Am. Academy of Dermatology, 43, 610–618 (2000).
3. G.J. Nohynek and H. Schaefer, Regulatory Toxicology and Pharmacology, 33, 285–299 (2001).
4. J.F. Nash, Dermatologic Clinics, 24, 35–51 (2006).
5. F.P. Gasparro, M. Mitchnick and J.F. Nash, Photochemistry and Photobiology, 68, 243–256 (1998).
6. European Directive 76/768/EEC and its successive amendments, basic act 31976L0768.
7. S. Schauder and H. Ippen, Contact Dermatitis, 37, 221–232 (1997).
8. B. Berne and A.M. Ros, Contact Dermatitis, 38, 61–64 (1998).
9. N. Cook and S. Freeman, Australian J. Dermatology, 43, 133–135 (2002).
10. A. Chisvert and A. Salvador, Analysis of Cosmetic Products, A. Salvador and A. Chisvert, Ed., Elsevier, Amsterdam, The Netherlands, 83–120 (2007).
11. S. Simeoni et al., J. Pharm. Biom. Anal., 38, 250–255 (2005).
12. C.W. Klampfl and T. Leitner, J. Sep. Sci., 26, 1259–1262 (2003).
13. S.P. Wang and W.J. Chen, Analytica Chimica Acta, 416, 157–167 (2000).
14. C.G. Smyrniotakis and H.A. Archontaki, J. Chromatogr. A, 1031, 319–324 (2004).
15. L. Dencausse et al., Int. J. Cosmetic Sci., 30, 373–382 (2008).
16. J.C. Cardoso et al., Colloids and Surfaces B, 63, 34–40 (2008).
17. A. Salvador et al., Green Chemistry, 4, G57–G58 (2002).
18. D.J. Schakel, D. Kalsbeek and K. Boer, J. Chromatogr. A, 1049, 127–130 (2004).
19. A. Chisvert, A. Salvador and M.C. Pascual-Martí, Analytica Chimica Acta, 428, 183–190 (2001).
20. A. Chisvert, M.T. Vidal and A. Salvador, Analytica Chimica Acta, 464, 295–301 (2002).
21. A. Chisvert, M.C. Pascual-Martí and A. Salvador, Fresenius' J. Anal. Chem., 369, 638–641 (2001).
22. A. Chisvert, M.C. Pascual-Martí and A. Salvador, J. Chromatogr. A, 921, 207–215 (2001).
23. A. Chisvert and A. Salvador, J. Chromatogr. A, 977, 277–280 (2002).
24. A. Salvador and A. Chisvert, Analytica Chimica Acta, 537, 15–24 (2005).
25. K. Klein and I. Palefsky, Sunscreens, N.A. Shaath, Ed. Taylor and Francis Group, New York, USA, 353–383 (2005).
26. J.N. Miller and J.C. Miller, Statistics and Chemometrics for Analytical Chemistry, Ed. Pearson, Harlow, England, 5th ed., 124 (2005).

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













