Development of a System Suitability Test for Two-Dimensional Liquid Chromatography (2D-LC)
Verifying system performance is important when using chromatographic instrumentation for analyzing both known and unknown samples. Typically, system suitability tests (SSTs) are used to verify performance, which involves running an established method and comparing the results to pre-established acceptance criteria. As two‑dimensional liquid chromatography (2D-LC) becomes used more widely and in regulated laboratory environments, development and implementation of SSTs will be critical for successful routine use of the 2D-LC technique.
Although I have not written in the past about system suitability tests (SSTs) for the “LC Troubleshooting” column, they were mentioned several times in the past by John Dolan, the previous “LC Troubleshooting” columnist. The keyword “system suitability” was used in 11 prior “LC Troubleshooting” articles, which speaks to the importance of SSTs in the routine implementation of conventional one-dimensional liquid chromatography (1D-LC). As discussed by John many times, one benefit of regularly running SSTs is that the test results can provide early indications of a problem that is developing, which can be acted on by troubleshooting and solving the problem before it develops into a full-blown failure and results in instrument down time. However, I am not aware of any prior description of the development of a generic SST for two-dimensional LC (2D-LC) (although some are definitely expressing interest in this [1,2]), which we continue to see move in the direction of regulated laboratories, especially in the pharmaceutical industry. For this instalment of LC Troubleshooting, I asked Yehia Baghdady, Senior Scientist in Chemical Process Development at Bristol Myers Squibb, to share his experiences so far related to the development and implementation of a SST for routine use of 2D-LC for peak purity checks of small molecule separations in his laboratory. I’m hopeful that sharing these ideas and experiences will kick off a broader conversation around this topic in the community.
Dwight Stoll
One of the most attractive features of 2D-LC in the context of pharmaceutical analysis is the utilization of orthogonal (that is, complementary) selectivities in the first (1D) and second dimension (2D) separations. This feature is particularly valuable for the separation of structurally similar impurities that tend to be coeluted with the main compounds of interest in the pharmaceutical industry, such as active pharmaceutical ingredients (APIs), synthetic intermediates, and starting materials (SMs) (3–6). In these challenging separation scenarios, the peak capacity and resolving power of conventional 1D-LC is often insufficient—even for small molecules where the molecular complexity is lower compared to the challenges encountered in the analysis of large biomolecules.
Despite the recent increased availability of commercial 2D-LC instruments, 2D-LC technology is still not considered to be ready for routine use in quality control (QC) laboratories for small molecule in the same way that 1D-LC is (1). Transferability and robustness across different laboratories have not yet been demonstrated on the global scale, and 2D-LC also requires special hands-on experience, equipment, and software that are not widely available like they are for 1D-LC. Additionally, from a regulatory perspective, one or two independent 1D-LC methods are usually sufficient to provide the data needed to meet the analytical target profile of a small molecule pharmaceutical product. As a result, to the best of our knowledge there are no published articles in the literature providing guidelines about how to develop a periodic 2D-LC system suitability test (SST) capable of ensuring the quality of generated results and triggering early alarms for the need to replace a column or fix the instrument components before running real samples. Having a generic SST in place is important to enable routine use as well as to ensure consistent and desirable performance of 2D-LC as a whole integrated system.
Role of System SSTs for Chromatography Methods
The United States Pharmacopeia (USP) Chromatography General Chapter <621> provides guidance for the implementation of chromatographic methods in the pharmaceutical industry (7). It states:
Usually, analyte mixtures used in SSTs for conventional 1D-LC are designed to contain analytes that can be resolved with good peak shapes and acceptable symmetry or tailing factors (8,9). The situation with 2D-LC is different in the sense that some additional requirements need to be met. At a minimum, the mixture must contain two analytes that are well separated in the first dimension, but it is also helpful to have additional compounds that are both coeluted in the first dimension and separate in the second dimension. The behaviour of these particular compounds can be used to evaluate and monitor the performance of both separation dimensions at the same time. In addition to the implementation of this 2D-LC SST mixture to monitor system performance, the separation of peaks in the second dimension can help to initially adjust system parameters to improve the performance of the 2D separation before utilizing the 2D-LC system in routine pharmaceutical analysis. That is to say, selecting and examining the effect of various 2D chromatographic conditions, such as injection volume (volume of sampling loops), column dimensions, gradient, and flow rate on the peak shape, efficiency, and resolution, when collected 1D fractions of coeluted analytes are transferred to the second dimension.
Development of a SST Method for 2D-LC
Selection of Test Analytes: Selecting proper SST test analytes is essential to ensure the reliability and accuracy of the data generated by the 2D-LC system. Because the system is comprised of two dimensions integrated together through an online interface (single or multiple valves), the probes should be sensitive to the performance of both dimensions. We selected test analytes to fulfil the following criteria:
- Good UV absorbance (because the scope here is for 2D-LC systems with UV detectors only);
- Good solubility in common solvents;
- Commercially available at reasonable cost;
- Span a range of hydrophilicity and hydrophobicity that reflects the range of properties that might be encountered in future, real samples;
- Most importantly, respond differently to the selectivities used in the two-dimensional reversed‑phase separation
(for example, a pair of analytes were coeluted in the first dimension, but is separated in the second dimension).
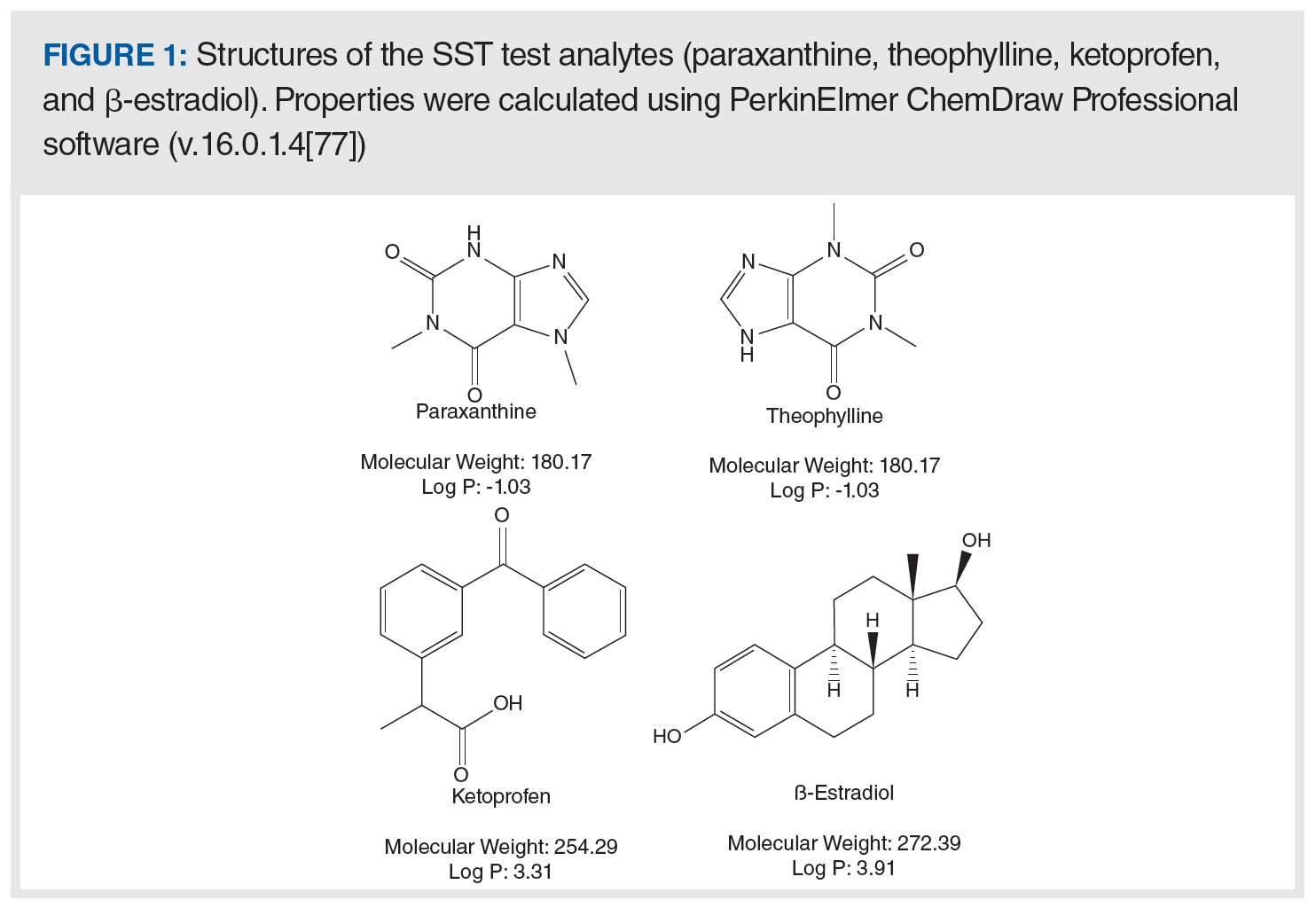
With these criteria in mind, a four-component mixture was designed such that two peaks are observed in the 1D separation, and each of the two peaks contains a pair of coeluted compounds. The structures of these molecules are shown in Figure 1, and representative chromatograms are shown in Figure 2. The first early eluting peak contains a coeluted pair of hydrophilic and highly polar compounds, whereas the late eluting peak contains a pair of coeluted hydrophobic compounds. The two hydrophilic compounds are structurally similar analogues that are difficult to separate, whereas the two hydrophobic compounds were chosen to be coeluted under the conditions of the 1D gradient. Generic chromatographic conditions (that is, flow rates, gradient slopes, and solvents) are used for both dimensions, and the two stationary phases used have complementary chemistries. The first is a traditional C18 column and the phase is used as a starting point in development of reversed-phase methods in our laboratory (that is, our platform method). The second is a polar‑embedded reversed‑phase column that is known to have selectivity highly complementary to most C18 columns (10,11).
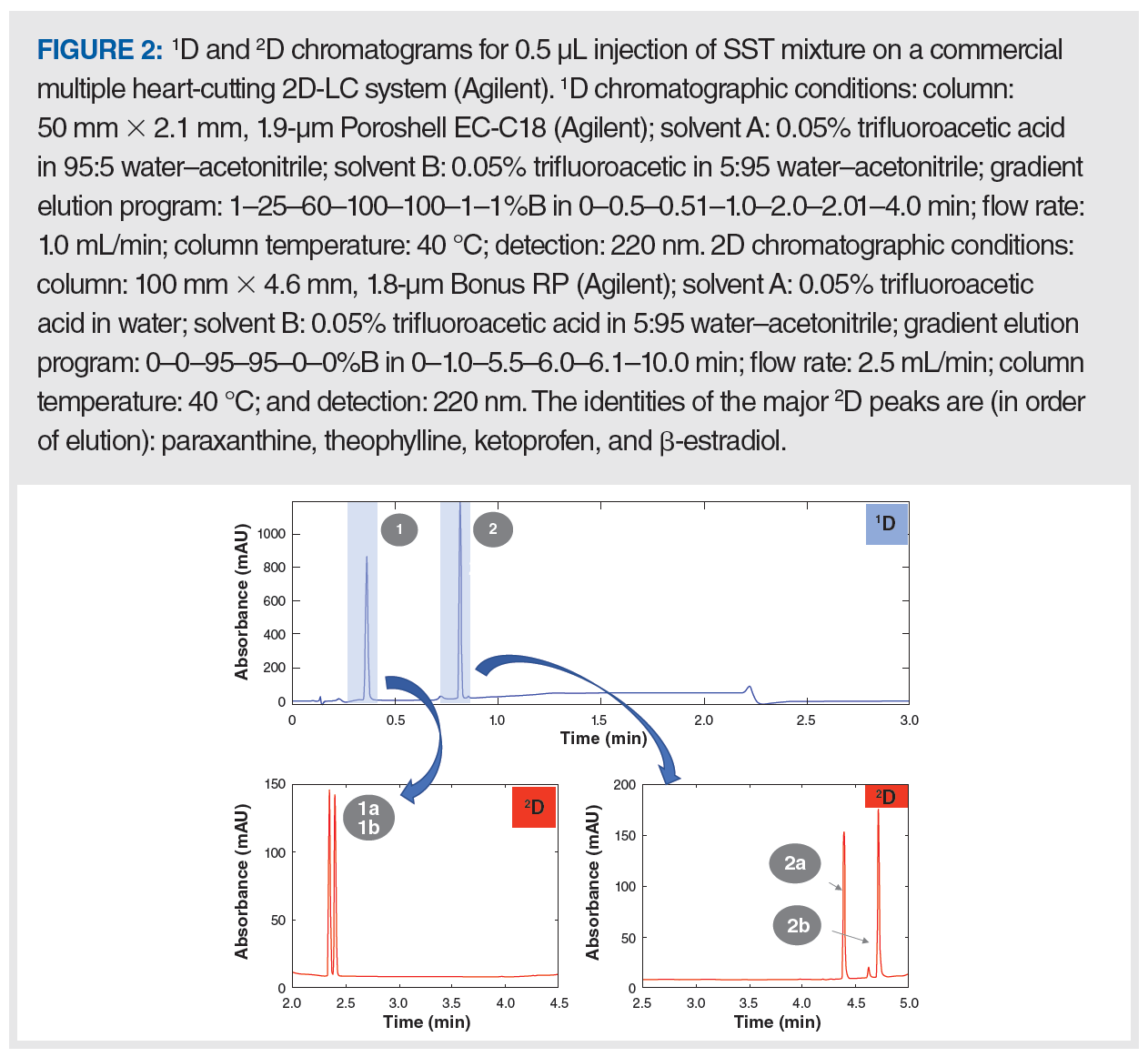
Establishment of Acceptance Criteria: The SST criteria are established to represent the minimum acceptable 2D-LC system performance rather than typical performance levels. This ensures that the predefined acceptance criteria are neither too wide—which would prevent detection of unexpected system problems—nor too narrow—which could trigger unnecessary alarms. The acceptance criteria we have settled on for this SST are as follows:
- The number of peaks within the selected integration time window—two 1D peaks in the 0.3–0.9-min window, and four 2D peaks in the 2.0–3.0‑min window for peaks 1a and 1b, and 4.0–5.0 min for peaks 2a and 2b;
- The relative standard deviation (RSD) of retention time (tR) and peak area <2% for triplicate injections;
- A USP tailing factor <2; and
- A USP resolution >1.5 for peaks in the second dimension.
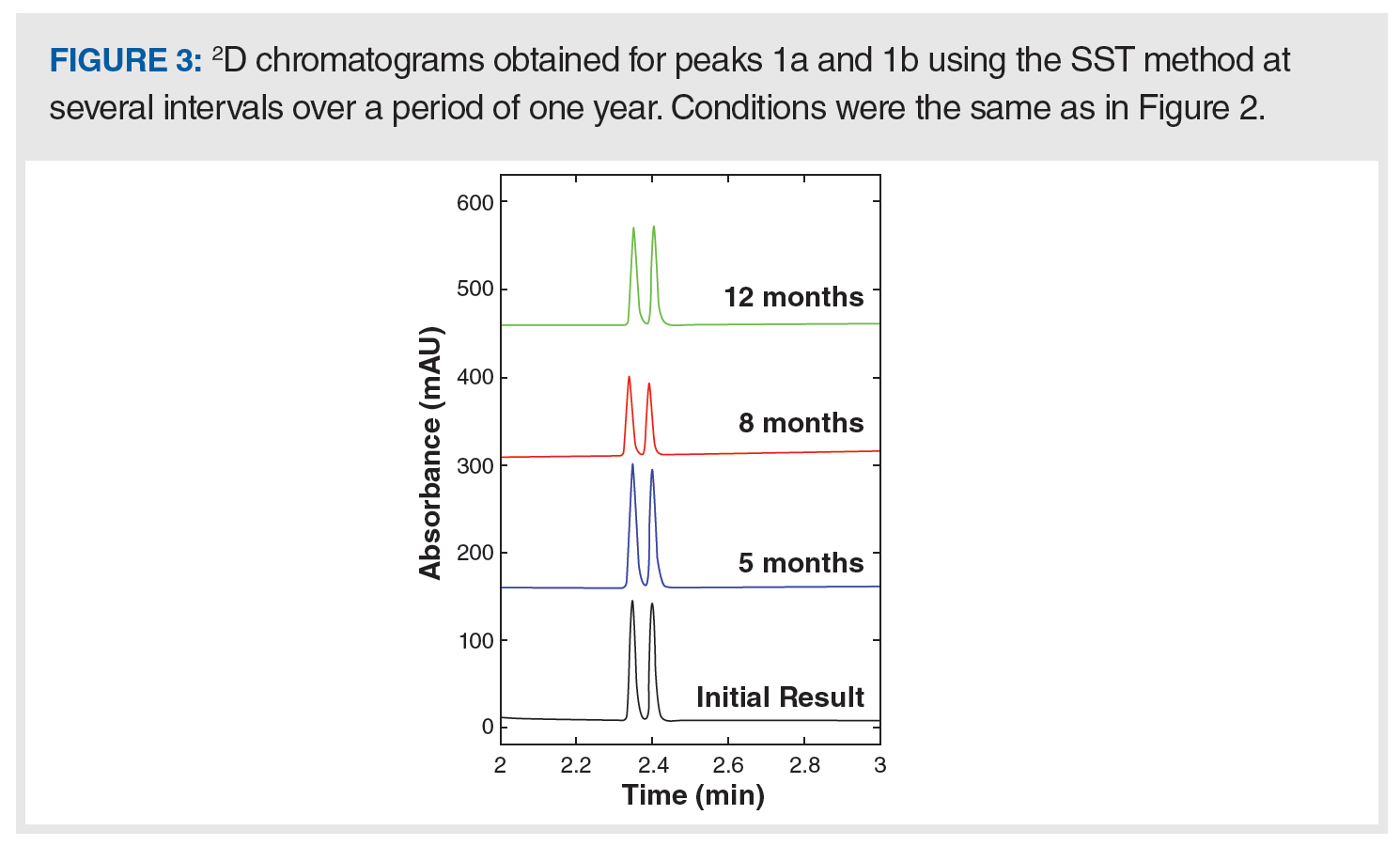
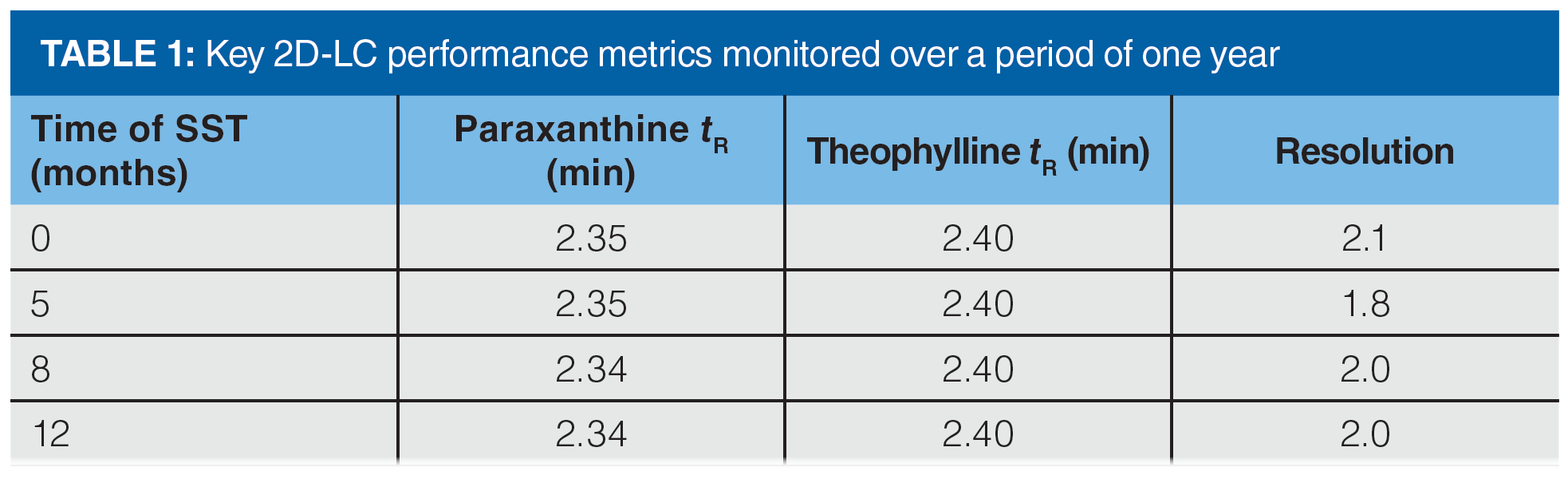
Performance of the SST So Far (One Year): We monitored the performance of our developed SST with respect to tR and resolution of the more challenging hydrophilic pair over the period of one year as shown in Figure 3 and Table 1. These data show consistent performance of the 2D-LC system for its intended purpose as a whole integrated system, and they show the convenience of this developed SST over an extended period of use.
Example of a Failed 2D-LC SST: Several 2D-LC SST failures could be attributed to various causes such as problems with the autosampler, interface valves, pumps, column ageing, and mobile phase preparation errors. The aforementioned acceptance criteria are sensitive to these errors and may trigger early alarms that prevent the acquisition of unreliable data that lead to inaccurate results and the need to retest samples. Figure 4 shows an example of SST failure in our laboratory where the 1D performance was out of specification. Figure 4 shows 1D chromatograms from two SST injections that illustrate a sudden significant difference in performance. With respect to the acceptance criteria, it is clear that the peak number check fails to meet the required number within the specified integration time window because of the large shift in tR of both peaks (blue trace in the chromatogram, where each peak represents a pair of coeluted peaks).
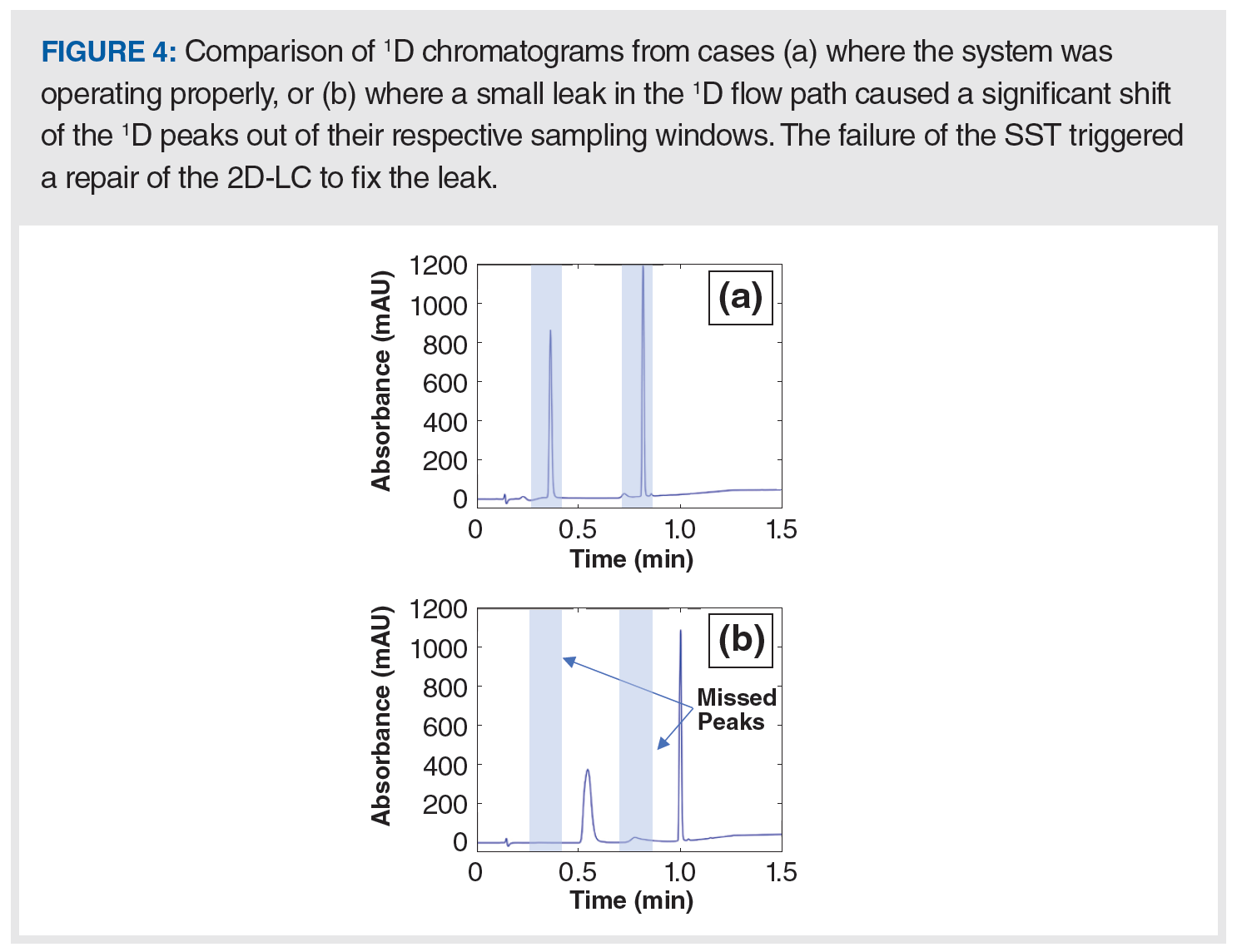
Investigating the cause of this problem resulted in the identification of a small leak in the pump B head, which delivers the organic solvent component of the mobile phase. The leakage was too small to trigger the built-in leak sensor of the pump. Therefore, if the SST did not reveal this issue, it would have passed undetected with negative consequences for the reliability of the 2D-LC result. In this context, a difference in 1D separation performance can result in coeluted impurities that otherwise separate, or vice versa. A notable point to mention here is that the peak shape was also another indicator of poor performance, which could be captured by either adopting peak efficiency (N) as an additional SST acceptance criterion or by the visual examination of the chromatogram. The peak shape (broader peak) and N for the coeluted hydrophilic pair was more affected than the later one because a small difference of the delivered gradient has a significant impact on the initial low fraction of organic solvent in the mobile phase (1%) that elutes polar analytes.
Summary
In this instalment of “LC Troubleshooting”, we described the development and implementation of a SST for multiple heartcut 2D‑LC. Such SSTs are routinely used for conventional LC systems to verify that the performance of the LC system is suitable for use in analyzing pharmaceutical samples. However, there has been little discussion of SSTs for 2D-LC in the literature to date. The 2D-LC SST described here is intended to serve as a starting point and will likely become more sophisticated over time as we learn more about the criteria needed for the most effective use of the SST. Initial results from the regular use of the SST over a period of one year both demonstrated consistent performance of the 2D-LC method and identified one instance where the system performance was not suitable because of a small leak in the 1D pump that was subsequently repaired to restore system performance.
Acknowledgement
The authors would like to thank Qinggang Wang for his feedback and suggestions while preparing this article.
References
- A. Clarke and A. Ehkirch, LCGC Europe 33(3), 136–150 (2020).
- S.H. Wang, J. Wang, and K. Zhang, J. Chromatogr. A 1492, 89–97 (2017).
- J.G. Shackman and B.L. Kleintop, J. Sep. Sci. 37, 2688–2695 (2014).
- D.R. Stoll and T.D. Maloney, LCGC North Am. 35(9), 680–687 (2017).
- C.J. Venkatramani, Supplement to LCGC Europe 31(s10), 22–29 (2018).
- Q. Wang, B.L. He, and J.G. Shackman, J. Pharm. Biomed. 193, 113730 (2021).
- General Chapter <621> “Chromatography,” in United States Pharmacopeoia (United States Pharmacopeial Convention, Rockville, Maryland, USA, 2022).
- M. Klarqvist, T. Leek, A. Babapour, and N. Falk, LCGC Europe 30(3), 110–118 (2017).
- I. Mutton, B. Boughtflower, N. Taylor, and D. Brooke, J. Chromatogr. A 1218, 3711–3717 (2011).
- Y. Zhang and P.W. Carr, J. Chromatogr. A 1216, 6685–6694 (2009).
- R.K. Lindsey, B.L. Eggimann, D.R. Stoll, P.W. Carr, M.R. Schure, and J.I. Siepmann, J. Chromatogr. A 1589, 47–55 (2018).
About The Co-Author
Yehia Z. Baghdady is Senior Scientist in the Chemical Process Development group at Bristol Myers Squibb, in New Brunswick, New Jersey, USA.
About The Column Editor
Dwight R. Stoll is the editor of “LC Troubleshooting”. Stoll is a professor and the co-chair of chemistry at Gustavus Adolphus College in St. Peter, Minnesota, USA. His primary research focus is on the development of 2D-LC for both targeted and untargeted analyses. Direct correspondence to: amatheson@mjhlifesciences.com

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).