Pyrolysis for the Preparation of Macromolecules and Analysis by Gas Chromatography
LCGC North America
When pyrolyzed, macromolecules will decompose into smaller fragments that can have the appropriate volatility for gas chromatography (GC) separation and analysis.
When pyrolyzed, macromolecules will decompose into smaller fragments that can have the appropriate volatility for gas chromatography (GC) separation and analysis. This decomposition is somewhat analogous to fragmentation in a mass spectrometer. Although the pyrolysis can be conveniently coupled to GC, the advances in other analytical techniques may have rendered pyrolysis-GC a somewhat forgotten technique to those who do not routinely work with polymers, biological molecules, and similar samples. This month, we take a look at the fundamentals and applications of pyrolysis-GC.
In most cases when we think of chromatographic sample preparation, we refer to extraction. If we consider that the overall goal of sample preparation is to get the analyte into a suitable form (in an appropriate solvent, free of interferences and sample matrix effects, and at a proper concentration), other techniques, including analytical pyrolysis, are often overlooked.
In a 2008 review, Kaal and Janssen (1) identified four standard approaches for extending the mass range of gas chromatography (GC): high-temperature GC, sample derivatization, pyrolysis, and thermochemolysis. Pyrolysis exploits the use of high temperatures, often under an inert environment, to decompose large molecules into smaller fragments representative of the molecular structure and is sometimes called thermal decomposition or thermal degradation. Officially, the International Union of Pure and Applied Chemistry (2) defines analytical pyrolysis as "the characterization, in an inert atmosphere, of a material or chemical process by a chemical degradation reaction(s) induced by thermal energy." Although other combinations of pyrolysis and instrumental analysis, notably pyrolysis–mass spectrometry (py-MS) and pyrolysis–infrared spectroscopy (py-IR) are important, this column focuses on pyrolysis–gas chromatography (py-GC). The historical development of analytical pyrolysis is presented in Table I with information gleaned from the Encyclopedia of Analytical Science (3).
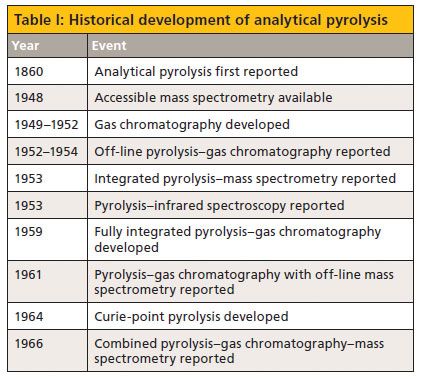
Table I: Historical development of analytical pyrolysis
As a sample preparation technique, pyrolysis enjoys several advantages, including speed, accommodation of small samples, and applicability where other analytical methods may be limited. However, by definition, it is a destructive technique and finding an appropriate internal standard is often difficult. But the technique has outgrown the initial perception of it as being a crude technique, which was likely because of the lack of precision with homemade systems and the residue (char) remaining after sample pyrolysis.
Pyrolysis Mechanisms
Reviews of developments in py-GC summarize the thermal degradation of polymers into three categories: random chain scission, side-group scission, and unzipping (4,5). Because all bond energies in a C-C backbone are equal, random scission along a polymer chain may occur, resulting in smaller fragments. From synthetic polymers, these smaller fragments are a mix of alkanes, alkenes, and dienes. During side-group scission, the side groups are cleaved from the polymer backbone. The resulting unsaturated polymer will then undergo rearrangement reactions. Side-group scission results when the bonds holding the side groups to the polymer backbone are weaker than the bonds comprising the polymer backbone. Unzipping is a depolymerization process resulting in decomposition to the monomer or, occasionally, short-chain oligomers. Thus, sample degradation is dependent on molecular structure and bond dissociation energy. Other degradations such as cross-linking, isomerization, cyclization, and char formation are possible. These breakdown reactions will proceed in order from cleavage of weaker bonds to stronger bonds. Pyrolytic bond breaking frequently results in free radical formation. Since free radicals are stabilized by recombination, quite often larger, rather than smaller, fragments may occur. The products of the thermal breakdown are related to structure, stability of the free radical, and subsequent rearrangements. Study of the pyrolysis mechanism for an individual compound is difficult and usually requires a step-wise programming of the pyrolysis temperature. If the pyrolysis temperature is too low, the degradation time may be too slow even if pyrolysis occurs. But if the temperature is too high, more complete degradation may occur, obscuring useful fragmentation of the analyte. Hence, temperatures of 500–800 °C are commonly used in py-GC–MS. Slow pyrolysis temperature programs (linear or stepwise) might be used to progressively uncover thermal fragmentations. Chemometric methods can be applied to assist in the interpretation of complex pyrograms (a pyrogram is the chromatogram resulting from py-GC).

WELCOME TO THE NEW EDITOR OF "SAMPLE PREP PERSPECTIVES"
As an example of these pyrolytic mechanisms applied to a complex sample, we can see the py-GC of kerogen. Kerogen is the insoluble, high molar mass organic matter found in sedimentary rocks, including oil shale. Figure 1 displays the pyrogram resulting from the py-GC–MS of kerogen showing the mixture of normal alkanes and alkenes systematically cleaved from the paraffinic material, presumably by the random scission mechanism.
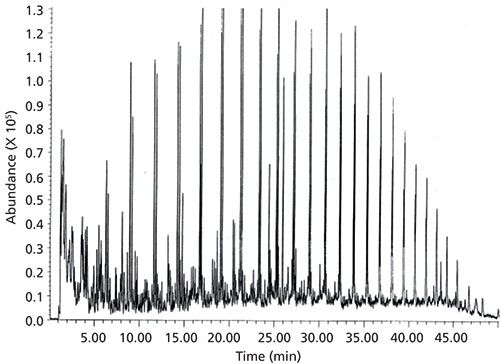
Figure 1: Pyrolysis–GC–MS of a kerogen sample displaying alkane–alkene doublet peaks. A filament pyrolyzer at 750 °C for 20 s was used. Adapted with permission from reference 1.
Types of Pyrolyzers
In commercial systems, three types of pyrolyzers predominate: microfurnaces, Curie-point pyrolyzers, and filament or ribbon pyrolyzers. In microfurnaces, samples are injected via syringe or are directly placed into the pyrolyzer cup. The pyrolysis zone is isothermally heated. Microfurnaces represent the slowest and least reproducible pyrolzer used with GC. On one hand, the slow heating leads to the possibility of secondary reactions; on the other hand, the sample amount and its position within the cup are less of a concern. Because the pyrolysis cup in a microfurnace is continually heated, care must be taken during sample introduction. Microfurnaces are perhaps the most appropriate for nonpowder solids.
Curie-point pyrolyzers have a coil made from a ferromagnetic metal, which is heated rapidly using a high frequency generator. The design of Curie-point systems allows for very rapid heating to the final temperature, which is dependent on the Curie point (that is, transition from ferromagnetic to paramagnetic) of the metal used. Final temperatures may vary from 400 °C with a 45:55 nickel–iron alloy, to 770 °C with iron, and up to 1128 °C with cobalt.
Platinum or other resistive metals allow programmable temperature ramps in filament (also called ribbon) pyrolyzers. Similar to Curie-point pyrolyzers, filaments allow for extremely fast heating, but also allow for a wide temperature range and temperature programming at a controlled rate. Because they provide fewer secondary reactions, pyrograms from Curie-point and filament systems are generally easier to interpret. Filament systems are the most commonly used, and versatile, pyrolyzers for py-GC. Other pyrolysis systems, including UV pyrolysis, have been explored. Notably, significant interest has centered on laser pyrolysis, but these systems have yet to be considered within the main stream of commercial instrumentation.
Practical Considerations for Pyrolysis-GC
As with any hyphenated technique, one must carefully consider the configuration and operating conditions of the individual analysis methods, as well as the interface. In py-GC, near instantaneous heating of the sample in the pyrolyzer provides more uniform heating and allows injection with minimal band broadening. Additionally, very rapid pyrolysis can avoid discrimination issues with samples covering a wide boiling point range. Discrimination results from an increased amount of more-volatile sample components being fed to the chromatographic column in preference to the less volatile components. Small samples, on the order of tens of micrograms with capillary columns, allow for effective heat transfer and minimize the potential of column overload. Because organic matter is generally a poor heat conductor, sample size and distribution within the pyrolyzer are important. Of course, appropriate sampling considerations must be employed to ensure homogeneous and representative samples are used, which is a valid concern with the small sample sizes used in py-GC.
The pyrolyzer and its interface to the GC must be swept efficiently to avoid exposure to hot or cold spots and to minimize band broadening because of dead volumes. This means that the volume of the pyrolyzer must be minimized and evacuated efficiently during and after the pyrolysis reaction. Thus, carrier gas flow must be reasonably high, typically in the 30–40 mL/min range. Because of this high flow rate, as well as for sample capacity considerations, split injection is typically used. Cryogenic focusing is also commonly used to focus the injected pyrolysate onto the head of the GC column. Although the microfurnace pyrolysis approach is the easiest way to handle solid samples, generally samples are introduced to the pyrolyzer in solution or coated as a thin film onto the heating element. Insoluble materials can be finely ground and introduced as a suspension or a powder.
Applications
Py-GC traditionally is applied for the analysis of synthetic polymers and biological macromolecules. More recent developments have greatly expanded the field, and there are a number of reviews available to present specific cases. In forensic applications, for example, samples can be compared and differentiated (so-called chemical fingerprinting) with or without complete compositional or structural analysis. Especially with mechanistic knowledge of the sample pyrolysis, structural determination and sample composition and identification can be made. Also specialty applications, such as chemotaxonomic characterization of microorganisms, are becoming increasingly commonplace. Any conventional GC detector can be used with py-GC, though mass spectrometry seems to be the most widely used because of the unique combination of universality and selectivity offered by MS. While the application areas of py-GC continue to grow, typical applications of the technique fall in the characterization and analysis of the following samples:
- plastics and polymers,
- atmospheric particulates,
- biological macromolecules such as proteins, lignin, polysaccharides, resins, and adhesives,
- fiber analysis from clothing and carpeting,
- paints, varnishes, glues, and finishes,
- and bacteria and other microorganisms.
Thermochemolysis
The Kaal and Janssen review (1) identified thermochemolysis as an additional approach to the GC analysis of high molar mass samples. In actuality, thermochemolysis is a pyrolysis technique combined with in situ derivatization. Commonly, tetramethylammonium hydroxide (TMAH) is used to form volatile methyl esters of oxygenated materials like carboxylic acids or hydroxyls. This approach extends the realm of py-GC into more-polar samples with a technique that is rapid and requires little sample pretreatment. Other derivatization agents allow for hydrogenation, methylation, and silylation reactions. The pyrolysis or derivatization with TMAH occurs at lower temperatures than conventional pyrolysis, minimizing the occurrence of secondary reactions. Notably, standard py-GC systems can be used if the sample is mixed thoroughly with the derivatization reagent.
Conclusion
While often considered a mature technique, analytical pyrolysis, especially when coupled with GC, MS, and IR, still sees growth in application areas. For the past 35 years, the Journal of Analytical and Applied Pyrolysis has reported advancements in the field, though general analytical chemistry and chromatography journals also present research in analytical pyrolysis. Although most laboratories will continue to rely on various extractions for the bulk of their sample preparation before chromatographic analysis, it is worth keeping py-GC in mind for those cases where conventional approaches are less suited.
References
(1) E. Kaal and H.-G Janssen, J. Chromatogr. A1184, 43–60 (2008).
(2) P.C. Uden, J. Anal. Appl. Pyrol. 31, 251–256 (1995).
(3) T.P. Wampler, Encyclopedia of Analytical Science (2nd ed.) (Elsevier, 2005), pp. 81–95.
(4) T.P. Wampler, J. Chromatogr. A842, 207–220 (1999).
(5) K.L. Sobeith, M. Baron, and J. Gonzalez-Rodriguez, J. Chromatogr. A1186, 51–66 (2008).
"Sample Prep Perspectives" editor Douglas E. Raynie is an Associate Research Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee.

Douglas E. Raynie

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.








