Challenges in the Determination of Protein Aggregates, Part I
LCGC North America
Most analytical approaches used for the determination of low-molecular-weight noncovalent aggregates in protein pharmaceuticals suffer from important limitations. This first part of this two-part column series discusses those limitations.
Most analytical approaches used for the determination of low-molecular-weight noncovalent aggregates in protein pharmaceuticals suffer from important limitations. This first part of this two-part column series discusses those limitations. Part II will present a viable analytical approach that offers, in our opinion, the best solution for analyzing these aggregates.
The intent of this column is to address what analytical approaches and instrumentation can be used for accurate, precise, qualitative, and quantitative determination of low-molecular-weight noncovalent aggregates of proteins. These aggregates are often found in biopharmaceutical formulations and commercial products, at varying levels or percent compositions (for example, x% monomer, y% dimer, and z% trimer). This first part of this two-part column series will discuss the limitations of most methods commonly used for this analysis. In part II of this series (coming out in June 2015), we will put forth a viable analytical approach that offers, in our opinion, the best solution for analyzing these aggregates.
Limitations for Characterizing Protein Aggregates
There are many separation technologies commonly used today that are capable of partially characterizing protein aggregates. These include size-exclusion chromatography (SEC), sodium dodecyl sulfate (SDS)-gel electrophoresis, polyacrylamide gel electrophoresis (non-denaturing) (PAGE), two-dimensional (2D) gel electrophoresis, differential gel electrophoresis (DIGE), high performance SDS-capillary gel electrophoresis (SDS-CGE), and field flow fractionation (FFF). Some of these tools can be very useful in resolving individual species, such as monomers, dimers, and trimers. However, as we hope to show in this column installment, none of these techniques alone really provide an accurate, precise, and fully reliable determination of the qualitative and quantitative nature of aggregates that may be present in a protein drug substance (DS), a protein drug product (DP), or a research protein. Even with authentic reference materials or calibration standards for a given protein, none of these separation methods has been shown to be 100% accurate in identifying the exact higher-order species present in a given sample or the absolute amounts of each in that sample.
The fundamental problem is that high-resolution techniques such as multiangle light scattering (MALS), right-angle light scattering (RALS), low-angle laser light scattering (LALLS), viscometry, mass spectrometry (MS), tandem MS (MS-MS), single or selective reaction monitoring (SRM), native MS, ion mobility spectrometry (IMS), and microflow imaging work best when first interfaced with some sort of separation tool - such as SDS-CGE, ultrahigh-pressure liquid chromatography (UHPLC), SEC, or FFF - but these separation tools may alter the sample after introduction. Such alterations of sample usually occur by dilution (in the run buffer) or as a result of selective adsorption onto the stationary phase of the individual proteins or aggregates. In the case of macromolecular protein complexes (higher-order species rather than simple monomers, dimers, or trimers), MS has generally been shown not to degrade or change the nature of the protein complexes in solution (1–3). However, this resistance to degradation in MS does not apparently apply to low-molecular-weight aggregates, which are often found in biopharmaceutical formulations (4,5).
If MS is used by itself, one may be able to avoid the sample alteration that can result from dilution, ionization, and gas-phase effects. However, we are not aware of any studies using a direct MS detection step that can unequivocally determine the nature and levels of low-molecular-weight noncovalent protein aggregates with a high degree of confidence. In most SEC–MS analyses, higher-order complexes tend to dissociate. In addition to the quantitation of aggregates that may be present in the original sample, determination of the species distribution (x% monomer, y% dimer, z% trimer, and so on) is also required. Aggregates may not ionize to the same degree in various modes of MS. In addition, there are no authentic reference standards with which to compare a sample and aggregates may dissociate under high vacuum from dilutional effects in the gas phase (for example, in IMS or macro-IMS), and might change the equilibrium from what was present in the original sample.
Some of the questions we hope to address in this two-part series include
- What are our concerns about using the various separation methods to identify and quantitate protein aggregates, regardless of the detection scheme? (Part I)
- What are the most common detection schemes usually interfaced with separation methods to identify and quantitate protein aggregates? (Part I)
- What data are generated using MALS with or without SEC–FFF as the first separation step? Is direct (or batch) MALS or concentration gradient (CG) MALS a feasible approach to characterize aggregates, either qualitatively and quantitatively? (Part II)
- Of the most common detection schemes for aggregates, which appear capable of determining aggregates with a high degree of confidence and assurance, both qualitatively and quantitatively? And, which do not change the nature of the original sample after introduction? (Part II)
- How do static or dynamic light scattering fit into the analysis of low-molecular-weight aggregates? (Part II)
- What is the best possible approach for future determinations of protein aggregates (qualitatively and quantitatively) and why? (Part II)
SEC–MALS as an Example
To some extent, two of our previous "Biotechnology Today" installments are relevant to the discussion here because they dealt with using light-scattering detection with SEC and high performance liquid chromatography (HPLC) for protein and antibody studies (6,7). However, the intent of this installment is to highlight the deficiencies in the commonly used analytical methods for qualitative and quantitative determinations of protein aggregates. Nevertheless, what was presented in 2012 is still pertinent. We believe that any separation method may well change the nature of the aggregates in ways that cannot be predicted beforehand, and that such approaches, even SEC, must be considered as potentially artifact prone (8–18). We hasten to add that there is a substantial library of excellent previous publications that have, in part, dealt with this very area, including for SEC–MALS and beyond (19–24).
There are, at least, two fundamental and widely applied ways to determine the nature, molecular weight, and sizes of protein aggregates in any formulation or mixture of these, together with the protein pharmaceutical of interest. The most commonly used method involves separation–detection with a wide variety of both separation and detection schemes, as mentioned earlier. The second is the direct or batch approach, in which no separation step is involved and the sample is just subjected to the detection scheme of interest, such as MALS, sedimentation velocity analytical ultracentrifugation (SV-AUC), viscometry, MS, or others. Each of these approaches has advantages and disadvantages, but the major issue is which of these tools are likely to alter the nature of the sample components during the measurement steps required.
Here, we consider SEC, but what is discussed also applies to FFF, CGE, DIGE, PAGE, IMS, and microflow imaging (MFI). There are some excellent overviews of the current approaches for the determination of protein aggregates, including SEC, FFF, and other separation schemes, which should be consulted (9–11) in view of what we are going to suggest. There is also a large number of application notes from all of the major SEC and HPLC vendors. In all of these studies, however, very little is discussed about how the separation methods may be altering or changing the sample before the detection step (12–18). They all seem to suggest that the final chromatogram, no matter what the detection scheme, represents what was initially present in the injected sample. However, it has been known for decades that various separation methods might dilute the injected sample or cause certain sample components to be adsorbed, reversibly or irreversibly, on the packing materials. This might happen because of ion-exchange, preferential adsorption of certain species, or partitioning into pores that monomers escape with ease. Thus, what the detector finally sees may not be what was in the original sample. What we suspect about chromatography may well also apply to electrophoretic approaches. After all, these too are, by and large, dilutional methods.
Now, let's turn to a few interesting studies on the use of SEC and MALS to resolve and then identify the precise nature of each peak present from insulin (25), bovine serum albumin (BSA), and hemoglobin (27–30). We have not been able to locate any refereed publications in standard journals that demonstrate the possible effects of dilution on the originally injected sample in SEC–MALS quite as clearly as Wyatt's application notes do. We should also indicate that the number of published studies intended to fully understand how low-molecular-weight aggregates actually behave in SEC and HPLC is very limited. There are many application notes, but few describe what actually happens to the protein sample after injection. The best such study, to our knowledge, is by a vendor of advanced instrumentation that is widely used to study protein molecular weight and structure, as well as these very protein aggregates - Wyatt Technology.
Hemoglobin and BSA
Figure 1 shows the SEC–MALS separation of hemoglobin (52–62 kDa) and BSA monomer (66 kDa) and dimer (132 kDa) (15). It is very clear that the hemoglobin has a wide range of species present (52–62 kDa) (15). BSA is a rather simple protein, mainly monomer and some dimer, with very good agreement in molecular weights. Other researchers have also shown that BSA commonly has other aggregates present, such as trimer and higher species (27–29). The order of elution in Figure 1 is typical of SEC–MALS studies, in that higher-molecular-weight species are eluted ahead of those of lower molecular weights, but it is really the shape (linear versus globular) and hydrodynamic size of the species that determine elution order rather than molecular weight. From such a typical chromatogram, looking at just BSA, the relative percent of the dimer to the monomer is easily calculated using ultraviolet (UV) or refractive index (RI) detection, but it is not clear that such compositions relate accurately to the solution before injection. One should recall that the nature and amounts of protein aggregates are functions of various factors, such as the molecular weight of the monomer, the concentration of the monomer, how the sample was stored, the nature of the formulation, and temperature. This has been true for virtually all SEC studies ever performed for the determination of percent aggregates present in the starting protein or antibody (27–30). However, what the SEC chromatogram tells us may have been affected by dilution or adsorption effects within the separation itself, perhaps unique to that protein mixture and column. Such separation effects have been speculated on in the literature for decades, but little has been done, experimentally, to define or fully demonstrate the extent of such effects for individual proteins. In general, such serious effects have been ignored.
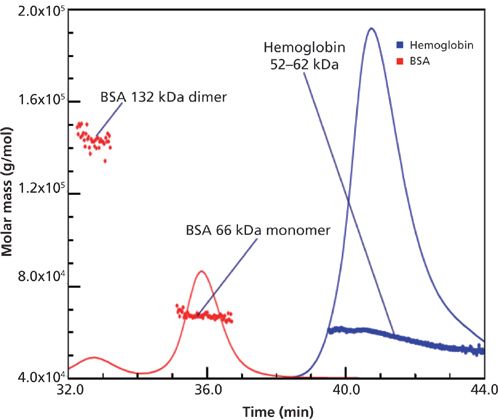
Figure 1: SEC–MALS separation of BSA and hemoglobin (proteins with similar molar masses). Adapted with permission from reference 15.
There are probably dozens of application notes, from a variety of vendors, that market detection and separation technologies for proteins and antibodies (3,11–13,30). The problem is that these methods are demonstrating what is coming out of the separation vehicle, and not necessarily what was present in the sample before injection. In SEC all data are relative to the performance of the sample relative to the standards. This includes dilution effects and mobile-phase composition. The result is an interpretation of molecular weight or apparent molecular weight (aggregates) and these data vary with each column. Any data need to be derived from a fresh molecular weight calibration curve for the SEC column as it exists at that moment, so calibration curves should be run both before and after the samples.
What this implies is that there is no absolute information one can obtain in SEC, only relative data. To claim otherwise does not appear to be valid. Even running SEC under sample storage conditions rather than with a dilution affects the result since hydrophobic interaction can increase from an increase in salt content, or ion-exchange effects can increase or decrease relative to the salt concentration or buffer pH.
Insulin
In another application note published by Wyatt in 2013, it was demonstrated that for a simple peptide such as insulin, if different volumes of the same sample solution were injected, different aggregates (or ratios of these) were observed (14). There are actually at least two different ways that such a study could be performed: with a single concentration and varying injection volumes or with different concentrations but injecting the same volume from each solution. In this study, the same concentration was injected in varying volumes and completely different SEC chromatograms and species were observed. Why were the resultant chromatograms so different from one another? Which set of results is valid, authentic representation of the original sample before injection?
This and other literature citations (31,32) were the motivation for this column. While references are available on the characterization of insulin aggregates using SEC combined with various types of detection, including MALS (25,33–34), none of them discusses the issue mentioned here. It is clear from the Wyatt application note that SEC is not an innocent bystander in the separation and identification of protein aggregates, even for samples as simple as insulin. Indeed, mainly by dilutional effects, SEC conditions can change the chromatogram just by changing the volumes injected of the very same insulin solution, a single concentration! Furthermore, different buffers may also play an important role in stabilizing or destabilizing insulin's aggregates in SEC. How do we then know which buffers, volumes, or concentrations to inject, if all of these factors may produce different chromatograms for the very same, protein sample injected, at least for insulin but perhaps for any or all proteins and peptides? Well, in fact, we don't.
Figure 2 illustrates injections of two different insulin formulations at the same approximate concentrations, and then analyzed by SEC–MALS. Sample 1 shows a stable monomer–dimer equilibrium with a small fraction of hexamer, while sample 2 shows monomer–dimer equilibrium in conjunction with a stable hexamer. The hexamer in sample 2 is present at much higher levels relative to the monomer–dimer than in sample 1. The same volumes were injected in Figure 2. Thus, different formulations may exhibit totally different species and percent compositions. This fact has been known and observed countless times for numerous proteins or antibodies. We should remember that protein storage and running buffers in SEC have different stabilizing or destabilizing effects on protein aggregates. Also, the fact that injecting different volumes of the same sample solution can produce different chromatograms has also been known, or at least suspected. And, of course, different concentrations of the same protein can lead to the presence of different aggregate species, since which aggregates are formed is heavily determined by the molecular weight of the monomer and the final concentration of monomer injected into the SEC column. However, these results for insulin in SEC–MALS strike at the very heart of an important question: How can we continue to use SEC, FFF, or other common separation conditions to indicate the nature and relative amounts of protein aggregates in the original sample being introduced?
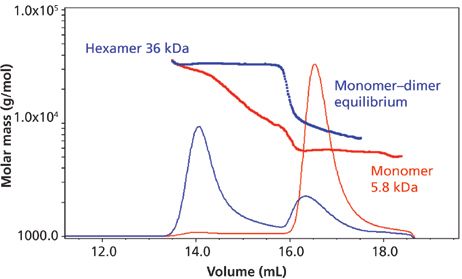
Figure 2: Molar mass versus time of insulin formulated in two different buffers, as determined by SEC–MALS. Adapted with permission from reference 14.
A Closer Look at the Problem
Let's look at the Wyatt application note on insulin aggregation (14) a bit more closely. Figure 3 illustrates the use of a single concentration of the same insulin sample, but using different injection volumes (50 versus 200 μL). The plot is that of volume (x-axis) versus molar mass (g/mol) (y-axis), which is customary in most SEC–MALS studies. What one clearly observes are different ratios of hexamer to the monomer–dimer equilibrium peak (they are not separable under these conditions because of their rapid equilibrium). In the case of the 50-μL injection, this is diluted substantially more in the SEC process than the 200-μL injection (fourfold), which causes the hexamer and dimer to dissociate more. It also seems obvious that in the last eluted peak in the 50-μL injection, since there is now more of the monomer than dimer, the peak corresponding to the lowest-molecular-weight species shifts to the right (is eluted later). It is also the case that if one measures just peak heights for the two peaks for each injection of Figure 3, the ratio of hexamer to combined monomer–dimer is greater for the 200-μL (3.53) injection than for the 50-μL (3.2) one. This again suggests more loss of higher-molecular-weight species at the lower volume injection because of greater dilutional effects. However, if the species involved were perfectly stable, as opposed to possibly equilibrating, noncovalent aggregates, then these changes would not appear when we inject different volumes of the same concentration.
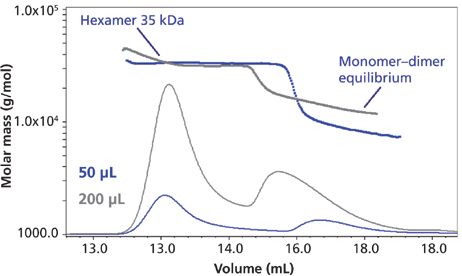
Figure 3: Molar mass versus time of the insulin sample from Figure 2, showing the concentration-dependent behavior of the monomer–dimer equilibrium assessed by SEC–MALS. Two injection volumes (50 μL and 200 μL) lead to different, overall concentrations of aggregates in the SEC column, resulting in different ratios of hexamer to dimer–monomer fractions and different monomer–dimer ratios. Adapted with permission from reference 14.
We could have observed similar results just by injecting the same volumes of different concentrations of the starting insulin. Recall that the resultant ratio of aggregates is a function of the molecular weight of the monomer, the buffer, and the concentrations or volumes of the same concentration being injected. This implies that we can observe any ratio of hexamer to monomer–dimer species just by varying the volumes injected for a single concentration or the same volumes for different concentrations. Or, if we use different formulations or different buffers, the final SEC–MALS result may also be entirely different. Thus, it is impossible to know for certain what the ratio of aggregate to monomer was in the original sample (solid state) or any given solution used for injections in SEC or in the original formulation. Nearly all of the existing approaches commonly used today suffer from this anomaly. It seems that the only way around this problem is to not use any dilutional separation step before MALS. That usually entails using a direct-MALS approach, also known as batch-MALS.
Other researchers have also demonstrated that increasing the concentration of a monomeric protein very often leads to the formation of more and more higher-order species, be these aggregates or oligomers (31,32). We should recall that protein associates or aggregates are different than oligomers, in that the first are noncovalent in nature and readily interconvert as a function of concentration, molecular weight, temperature, and time. Oligomers, on the other hand, are covalent structures that do not readily interconvert once formed, and they are not really in equilibrium with other oligomers or aggregates.
LptA is a naturally occurring plant protein that has been well characterized in the past (31). In this particular study, it was shown that LptA readily associates into stable, end-to-end oligomers even at relatively low, local protein concentrations. It was also shown that LptA forms a continuous array of higher order oligomeric, end-to-end structures as a function of increasing protein concentration. That is, as suggested earlier for the case of insulin (which forms only aggregates), this is another protein that forms varying mixtures of oligomeric structures just by changing the starting concentration of the protein. However, these oligomers are really disulfide covalent structures that do not interconvert under biological conditions to the monomer or other oligomers, once formed. Thus, there is not a dynamic equilibrium here (as with insulin), which apparently only forms noncovalent associates or aggregates that are all interconvertible with the monomer itself.
What seems strange is the general lack of literature reports on the artifactual or artificial formation of higher-order aggregates of proteins, as mentioned in the examples cited above and others. Surely, insulin is not the only bad actor that shows these effects of changing concentrations injected or volumes of the same concentration, as above. It seems that researchers, in general, have just not looked into these artifacts or they don't want to know they are happening with their particular protein sample.
Conclusions
Part I of this two-part article series highlights the inadequacies of some of the commonly used analytical methods that are used for quantitative and qualitative estimation of protein aggregates. In part II of this series, we will propose a possible solution to the above dilemma. There are several possible approaches. If it is clear by injecting different concentrations or different volumes of the same solution, that SEC–MALS chromatograms are changing (as above), then probably any method involving a combination of a separation plus MALS might be best avoided. And if one is to use a nonseparation-based method then various combinations such as SV-AUC, CG-MALS, batch-MALS or batch-dynamic light scattering (DLS), or batch viscometry, MS, or IMS and so forth could be useful. However, at the moment, it does not appear that there is any simple (nonseparations-detection) method that might provide accurate and precise percent compositions of monomer, dimer, trimer, and so forth, as easily and simply as SEC–MALS has apparently provided in the past, albeit with some artifacts, at times.
Acknowledgments
The authors wish to acknowledge several people within the Wyatt organization for their help and guidance in preparing this "Biotechnology Today" column, especially Dan Some and John Champion. The figures used are with the permission of Wyatt Technology. We appreciate the direct assistance of Mr. Cliff Wyatt. We acknowledge with appreciation John Philo of Alliance Protein Laboratories in Thousand Oaks, California, for providing us with copies of some of his publications on topics discussed in this column.
References
(1) I.S. Krull, S. Kreimer, and A.S. Rathore. LCGC North Am. 30(9), 842–849 (2012).
(2) S. Kreimer, J. Champagne, A.S. Rathore, and I.S. Krull, LCGC North Am.30(12), 1038–1045 (2012).
(3) "Size Exclusion Chromatography for Biomolecule Analysis, A How-To Guide," Agilent Technologies, Inc., 2013, 5991-3651EN.
(4) T. Arakawa, J.S. Philo, D. Ejima, K. Tsumoto, and F. Arisaka, BioProcess Int. 32–42 (November 2006).
(5) T. Arakawa, J.S. Philo, D. Ejima, K. Tsumoto, and F. Arisaka, BioProcess Int. 36–37 (April 2007).
(6) T. Arakawa, J.S. Philo, D. Ejima, H. Sato, and K. Tsumoto, BioProcess Int. 52–70 (November 2007).
(7) P.T. Duong and J. Martosella, "Separation of Recombinant Erythropoietin (repo) Using Agilent Bio SEC-3," Application Note, BioPharma, Agilent Technologies, Inc., 2011, 5990-9544EN.
(8) L. Lloyd and K. Mapp, "Defining the Optimum Parameters for Efficient Size Separations of Proteins," Application Note, Biopharma, Agilent Technologies, Inc., 2011, 5990-8895EN.
(9) L. Lloyd, LCGC North Am.32(s4), 30–35, (2014).
(10) P. Hong, S. Koza, and E.S.P. Bouvier, J. Liquid Chromatogr. & Related Technologies, 35, 2923–2950 (2012).
(11) S. Koza, M. Lauber, and K.J. Fountain, "The Analysis of Multimeric Monoclonal Antibody Aggregates by Size-Exclusion UPLC," Waters Corporation, Application Note, 720004713EN, May, 2013.
(12) "Analysis of Monoclonal Antibody and Protein Aggregates Induced by Denaturation Using a Novel Size Exclusion Chromatography Column," TSKgel, Application Note, TosoHaas Corporation.
(13) M. George, H. Gu, T. Wu, and X. Huang, "Size Exclusion Columns for Tough Sample Separation," Sepax Technologies, Inc., Newark, DE, www.sepax-tech.com.
(14) P. Wahlund, D. Roessner, and T. Jocks, "Identification of Insulin Oligomeric States Using SEC-MALS," application note, Wyatt Technology, Santa Barbara, California, 2013.
(15) "Hemoglobin Characterization," Application Note, Wyatt Technology, Santa Barbara, California, 1999.
(16) S. Vahidi, B.B. Stocks, and L. Konermann, Anal. Chem. 85(21), 10471–10478 (2013).
(17) I-O. Ebong, N. Morgner, M. Zhou, M.A. Saraiva, S. Daturpalli, S.E. Jackson, and C.V. Robinson, PNAS108, 17939–17944 (2011).
(18) Y. Xuan, F. Debaene, J. Stojko, A. Beck, A.V. Dorsselaer, S. Cianferani, and M. Bromiski, "Monoclonal Antibody and Related Product Characterization Under Native Conditions Using a Benchtop Mass Spectrometer," Thermo Fisher Corporation, Application Note 597 (2014).
(19) I.S. Krull, R. Mhatre, and J. Cunniff, LCGC 12(12), 914 (1994).
(20) I.S. Krull, R. Mhatre, and J. Cunniff, LCGC 13, 30 (1995).
(21) V.K. Sharma and D.S. Kalonia in Aggregation of Therapeutic Proteins, W. Wang and C. Roberts, Eds. (John Wiley & Sons, Hoboken, New Jersey, 2010), chapter 5.
(22) N. Kapoor, R. Gupta, S.T. Menon, E. Folta-Stogniew, D.P. Raleigh, and T.P. Sakmar, J. Biol. Chem. 285(41), 31647–31660 (2010).
(23) J.A. Merten, K.M. Schultz, and C.S. Klug, Protein Science 21, 211–218 (2012).
(24) J.S. Philo, AAPS Journal8(3), E564–E571 (2006).
(25) J.S. Philo, Curr. Pharma. Biotech.10, 359–372 (2009).
(26) J.S. Philo in Integrated Approaches to Protein Aggregation and Particles, H.-C. Mahler and W. Jiskoot, Eds. (John Wiley & Sons, Inc., Hoboken, New Jersey, 2011), section III, chapter 13, p. 305.
(27) H.H. Stuting and I.S. Krull, Anal. Chem.62, 2107 (1990).
(28) I.S. Krull, R. Mhatre, and H.H. Stuting, Trends in Anal. Chem. 8(7), 260 (1989).
(29) H.H. Stuting, I.S. Krull, R. Mhatre, S. Krzysko, and H. Barth, LCGC 7(5), 402 (1989).
(30) "Analysis of Monoclonal Antibody and Protein Aggregates Induced by Denaturation using a Novel, Size Exclusion Chromatography Column," Application Note AN53, 0913, Tosoh Bioscience LLC, King of Prussia, PA, 2013.
(31) J.A. Merten, K.M. Schultz, and C.S. Klug, Protein Science21, 211–218 (2012).
(32) N. Kapoor, R. Gupta, S.T. Menon, E. Folta-Stogniew, D.P. Raleigh, and T.P. Sakmar, J. Biol. Chem.285(41), 31647–31660 (2010).
(33) A.K. Arun, C. Fernandez, and A.P. Minton, Biophys. Chem.148, 28–33 (2010).
(34) A.K. Attri, C. Fernandez, and A.P. Minton, Biophys. Chem.148, 23–27 (2010).
Ira S. Krull is a Professor Emeritus with the Department of Chemistry and Chemical Biology at Northeastern University in Boston, Massachusetts, and a member of LCGC's editorial advisory board.

Ira S. Krull
Anurag S. Rathore is a professor in the Department of Chemical Engineering at the Indian Institute of Technology in Delhi, India.

Anurag S. Rathore

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.












