The Potential for Portable Capillary Liquid Chromatography
Is the desired goal of “shrinking down” capillary liquid chromatography (LC) from large laboratory systems to accurate portable field instruments realistic? This article explores recent progress in the miniaturization of LC components-such as capillary LC columns, micro- and nano-flow pumps, detectors, and other essential system components-and the future outlook for operating capillary LC instruments in remote settings.
Liquid chromatography (LC) is one of the most widely used analytical techniques in the world. However, unlike many other chemical measurement technologies, it has not been “miniaturized” to the same extent. Over the past several years, a number of developments related to the preparation of columns on the capillary scale and the design of portable instrument components have made the goal of “shrinking down” LC more realistic. New approaches to the design of capillary LC columns, including improved packing strategies in fused silica tubular formats and the manufacture of microfabricated pillar array columns, have led to major advances in chromatographic performance. Micro- and nano-flow pumps, detectors, and other system components have been scaled down to be more compatible with these columns, while also creating the opportunity to operate instruments in remote settings. This recent progress in capillary LC, and the future outlook of the field, are discussed here.
Research in the area of capillary liquid chromatography (LC) has endured for more than 40 years (1). I began working in this area in the fall of 2009, when I joined the University of North Carolina at Chapel Hill as a graduate student under the direction of Professor James Jorgenson. It was around this time that he published an in-depth review on the topic of capillary ultrahigh-pressure liquid chromatography (UHPLC) (2), a technique his research group established in the late 1990s and early 2000s (3–7). Since that article was published, new approaches to studying the design and performance of capillary LC columns were demonstrated, and significant progress in the design of miniaturized LC instrument components was made. My colleagues and I have recently discussed various aspects of capillary LC, both here in LC–GC (8,9) and elsewhere (10,11). The goal of this article is to provide an assessment of the implications of these developments on the future of portable capillary LC instrumentation.
Capillary LC Column Design
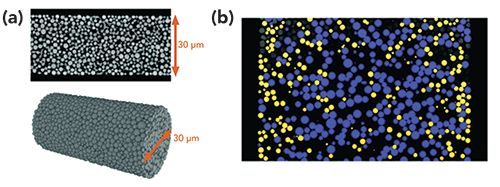
In recent years, fundamental investigations have helped improve our understanding of capillary LC column preparation (12–14). In 2010, the Tallarek group of Philipps-Universität Marburg demonstrated the ability to image the inside of capillary LC columns by utilizing confocal laser scanning microscopy (CLSM), from which a computational reconstruction of the bed structure could be generated and used to measure the column’s physical characteristics (15,16). Originally demonstrated for the characterization of monolithic stationary phase structures, the strategy was adapted to particle-packed beds as well (17). These original reports focused on bare silica, but an adapted technique using a hydrophobic fluorescent dye for the CLSM imaging process soon enabled the ability to image capillary columns packed with reversed-phase particles (Figure 1a) (18). This allowed for the first direct correlation between observed column efficiency and the morphology of the packed bed, providing new insight into the structural characteristics that influence chromatographic band broadening. Results showed that radial homogeneity of the packed bed plays a key role in maintaining high separation performance. A particle- size segregation effect was also observed in poorer-performing columns. Careful study of the bed morphology showed that smaller particles were more concentrated at the capillary wall and larger particles were more often found closer to the center of the column (Figure 1b). A number of additional studies were then conducted to better understand the cause of this effect, and to determine improved strategies for packing capillary LC columns.
Figure 1: In panel (a), a single CLSM image of a 30-µm i.d. capillary column packed with 1.9-µm particles and a full computational reconstruction of bed morphology derived from many of these images scanned axially through the capillary are shown. In panel (b), size segregation effects in a 75-µm i.d. capillary column packed with 1.9-µm particles in which smaller particles (in yellow) are concentrated at the column walls and larger particles (in blue) populate the bulk packing region. Adapted with permission from (18).
For many years, the approach to packing capillary columns had been to use dilute particle slurry concentrations as a way to reduce particle aggregation and promote a more ordered packed bed (19). However, experiments around the same time of the aforementioned column imaging studies indicated that packing with higher slurry concentrations provided better-performing columns than packing with lower concentrations (20). Comparisons of computationally reconstructed beds based on CLSM images of columns demonstrated that higher slurry concentrations mitigated the particle size segregation effect during column packing (21). However, very high slurry concentrations resulted in more voids in the packed bed structure that increased band broadening effects. These findings suggested an “optimal” slurry concentration that would simultaneously balance the effects of particle size segregation and void formation, a hypothesis that was later confirmed in studies conducted on a wide range of slurry concentration packing conditions for 75-µm i.d. columns packed with 1.3 µm (22) and 1.9 µm particles (23). Later findings revealed that slurry concentrations above the previously determined optimal level can be used to prepare very efficient columns if sonication is applied to the capillary during the packing process (24). Reduced band broadening and improved column-to-column performance repeatability were observed with this approach, with exceptional reduced plate heights of h = 1.05 achieved. These results demonstrate significant improvements in the preparation of highly efficient packed capillary LC columns, but challenges still remain in the quest for a general set of rules to produce ideal packed column beds. The “optimal” slurry concentration is different for every particle type and column aspect ratio (22), and axial heterogeneity can be a more difficult parameter to fully control (25). Also, many of these findings are specifically applicable to capillary-scale columns; studies to better understand the packing process of analytical-scale LC columns are ongoing (26–30). Finally, because these techniques require specialized, home-built equipment to facilitate the use of packing pressures in excess of 2000 bar, some researchers have instead utilized modified packing protocols with lower pressure requirements (31).
Other Miniaturized Column Formats
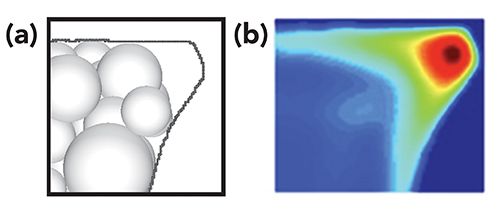
The development of packed LC columns in microfabricated devices has been demonstrated in a number of different formats for a variety of applications (32–35). Historically, the challenge with integrating particle-packed beds into microfluidic devices has been that widely used chip fabrication techniques, etching processes from two-dimensional planar designs, create channels with either semi-circular or rectangular cross-sections. In-depth studies on these geometries indicate that shapes containing sharp corners cause a dramatic loss in chromatographic efficiency (36–40). This is primarily due to the difficulty in achieving homogeneous flow profiles across the entire cross section because of the lower packing density that results from not being able to tightly fill these corners with spherical particles (Figure 2). Circular channels can be achieved, but very precise alignment strategies that are challenging to implement are required during device fabrication (41–43), as any minor misalignments can drastically exacerbate broadening. Monolithic columns have been used as an alternative stationary phase in microfluidic channels (44,45), a potential remedy to this issue as the stationary phase can better fill these void areas and reduce porosity differences in corner regions. However, current monolith column technology is most effective for the separation of larger biomolecules and typically exhibits lower plate counts than particle-packed beds for the separation of small molecules (46,47). Both particles and monoliths also suffer from additional issues when adapted to microfluidic platforms. Embedded channel structures often require a “world-to-chip” connection, which must hold pressures above those needed to flow mobile phase through the column at a reasonable linear velocity. A number of “clamp-like” strategies have shown promise for such a connection (48–52), with a specialized design capable of holding pressures of at least 1700 bar (53). Minimizing total chip footprint is another key to portability, which is often achieved by fabricating devices with serpentine channels. The addition of sharp turns in packed serpentine channels negatively impacts performance, especially for isocratic separations (53,54). Although the ultimate LC separation device may only be achievable with a completely integrated microfluidic instrument and column (55), many engineering hurdles still remain in the development of such a system.
Figure 2: Computational model of (a) random packing in a trapezoidal microfluidic device and (b) fluid dynamic modeling results on the cross-section of the model. The red color in the corner indicates differences in mobile phase velocity in this region compared to the rest of the packed channel. Adapted with permission from (40).
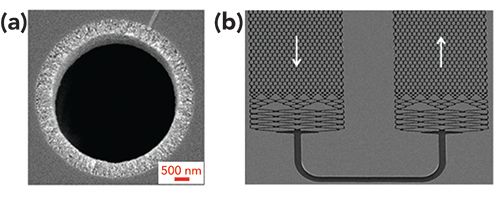
Other designs for miniaturized capillary-scale columns that provide for more highly-ordered stationary phase support structures can have advantages over packed beds and monoliths. Open-tubular LC (OT-LC) columns provide significant advantages to packed beds by eliminating any efficiency losses due to radial heterogeneity, because there is no packed bed structure (56). Advances in the preparation of these OT-LC columns in recent years have increased the efficiency that can be obtained using the technique (Figure 3a) (57,58), including their use for high-throughput and high-sensitivity separations (59,60). Ordered column structures can also be achieved using microfabricated pillar array columns (61), which can be designed in a variety of geometrical designs to optimize separation efficiency and reduce any potential impacts from wall effects that plague other chromatographic bed structures (Figure 3b) (62–65). The recent commercialization of this pillar array column format has greatly expanded its use for a wide variety of chromatographic applications, especially for the separation of biological molecules (66–69). The current limitation to adapting OT-LC and pillar array columns to portable LC is the decrease in sample loadability due to reduced stationary phase surface area. Although this area can be increased by adding porous monolithic structures onto fused silica walls (70) or etching the surfaces of pillar structures to increase porosity (71), detection modes that are most amenable to lower sample concentrations are still the main choice when using these types of columns. Mass spectrometry (MS) is often utilized, but there are a significant number of challenges when trying to reduce these large MS instruments to hand-portable formats (72). Electrochemical and fluorescence detectors are sensitive detection options that are more amenable to miniaturization (73–75), but they are only responsive to specific analyte classes unless analyte derivatization is performed (76). The wider utility of absorbance detection for many portable LC applications compared to these other techniques has limited the use of OT-LC and pillar array columns with miniaturized instruments to date, but this is a key opportunity area for future work.
Figure 3: SEM images of (a) an open-tubular capillary LC column with a porous monolith layer at the wall region and (b) a microfabricated pillar array column with flow distributors at the beginning and end of a turn region. Adapted with permission from (57,62).
Portable LC Instrument Design
When using capillary LC columns, the biggest challenge often lies with ensuring that the overall instrument system dispersion is low so that it does not significantly impact the separation performance (77–81). Reducing dead volumes in injectors, detector flow cells, and connections between instrument components is critical. Because of the challenges outlined in the preparation of completely microfluidic LC systems, miniaturizing instrument components so that the required fluidic connections and other sources of dispersion in the system are minimized has been a more widely adopted approach in recent years. An added advantage of shrinking down these components is that they can then be combined into integrated, portable LC systems. The key aspects of these systems are that they are small (both in weight and size), contain all necessary electronics and instrument components, can operate on battery or solar power for extended lengths of time, are simple to operate, generate minimal waste, and can achieve performance comparable to benchtop instrumentation (82). As this area of capillary LC instrument development has expanded, four main approaches have been pursued and are detailed here.
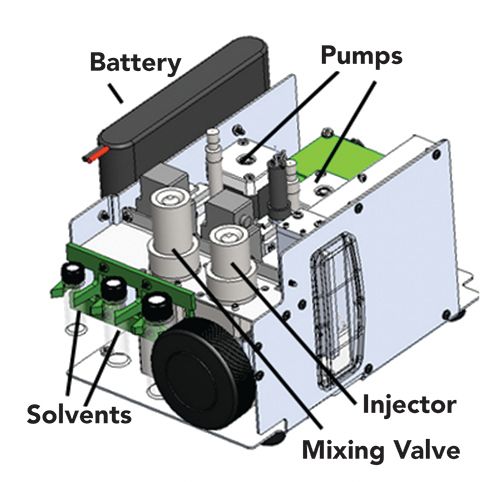
The Axcend Focus LC utilizes an integrated capillary column cartridge that can be inserted into a platform containing a pump and injector for tool-free column installation (Figure 4) (83). Flow is generated using two high-pressure syringe pumps that are capable of delivering capillary-scale flow rates up to 690 bar (84,85). The aqueous and organic solvents delivered individually from each pump are combined in a mixing valve. Samples are introduced into a four-port injection valve containing an internal loop in the 4-40 nL range that is compatible with the 150-µm i.d. columns that are incorporated into the cartridge, although the injection valve can be adapted to increase injection volumes up to 700 nL for methods that enhance gradient focusing of the sample at the inlet of the column. Inside the column cartridge, an on-capillary UV absorbance detector utilizing a light-emitting diode (LED) source is fixed directly at the column outlet to eliminate the need for post-column connecting tubing (86,87). A number of applications have been demonstrated using this instrument, including potency and impurity assays for over-the-counter (OTC) pharmaceutical drugs and illicit drug and drug metabolite monitoring (83). Dissolution studies on OTC products have also been performed, with retention time repeatability under 1% RSD across 50 chromatograms collected over 11 h. A prototype version of the platform has also been adapted for coupling to MS systems and used for protein studies (88). More recently, preliminary results for on-line synthetic reaction monitoring (89,90) and beverage quality control (91) were presented. As this system is the first commercially available portable capillary LC instrument, its use is expected to expand in coming years for applications that require remote analysis or the use of small footprint chromatographic instrumentation.
Figure 4: Schematic of the Axcend Focus LC. Adapted with permission from (83).
The Australian Centre for Research on Separation Science and the ARC Training Centre for Portable Analytical Separation Technologies at the University of Tasmania have reported a portable instrument design that uses modular components for LC separations at lower pressures (92–94). Commercially available syringe pumps with pressure limits around 100–120 bar are used for each individual solvent channel, which are combined in a mixing tee and sent to a micro-injection valve (95). A key difference with this instrument is the integration of more standard absorbance flow cells designed for commercial capillary-scale instruments with UV light-emitting diodes (LEDs) in a combined 3D-printed detector interface (Figure 5) (92,96,97). This approach potentially provides enhanced detection limits due to the increased flow cell path length, at the cost of increased detector volume compared to on-capillary detection. To reduce the effect of this larger detector volume on separation performance, the system has been optimized for use with 300-µm i.d. columns. The added benefit of this approach is that slightly higher flow rates can be used with 300-µm i.d. columns, which provided higher retention time reproducibility when using these pumps. Various versions of the system have been applied for the remote analysis of extracted plant materials (92) and environmental monitoring of anions in water using ion chromatography (94).
Figure 5: Diagram of a 3D-printed LED-UV detector cell, including 1) UV-LED source, 2) UV photodiode, 3) inlet for coolant flow, 4) outlet for coolant flow, 5) commercial flow cell insert, 6) coolant channel, 7) and 8) cell holders. Adapted with permission from (96).
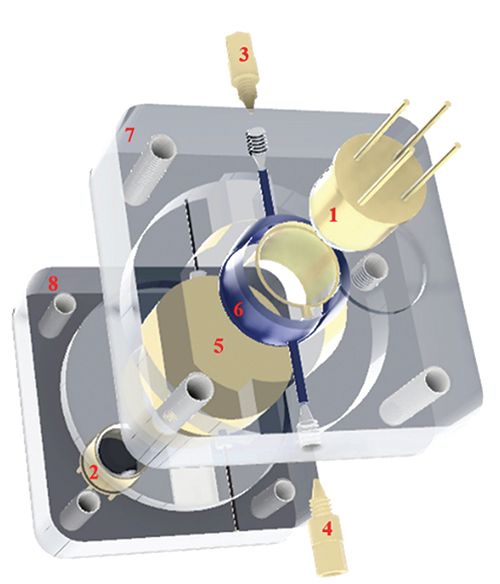
A third portable capillary LC instrument design, intended to allow for more rugged operation by using gas pressure to generate mobile phase flow, was recently described by the Salehi-Reyhani group (Figure 6) (98). This unique design provided a constant-pressure separation up to 150 bar, driven by a small gas cylinder controlled with a pressure sensor. Because the gas pressure pushes mobile phase from a large reservoir through the column, the length of a single run can be longer than when syringe pumps with a small chamber volume are used, although the use of the reservoir makes the generation of mobile phase gradients much more difficult. As with the two aforementioned portable LC instruments, this system uses an LED-UV absorbance detector. Both particle-packed and monolithic columns were tested with this gas-driven system, but fewer applications have been explored using this instrument, and thus far it has only been applied for the separation of two-component mixtures. The biggest advantage and most impressive result using this system is its robustness under harsh impact conditions. Because of its unique design, the only movable mechanical component is the sample injector, which ensures an added degree of stability compared to more traditional systems. The complete instrument was dropped three times from a height of 1 m while operating, and no significant baseline disturbance was observed any of the times it crashed into the floor. As vibrations might affect various mechanical components during transportation to remote locations and affect instrument operation for upon arrival, this approach helps minimize this potential issue in portable chromatographic analysis.
Figure 6: Schematic of a portable LC instrument platform integrating a gas pressure-based pumping mechanism. Adapted with permission from (98).
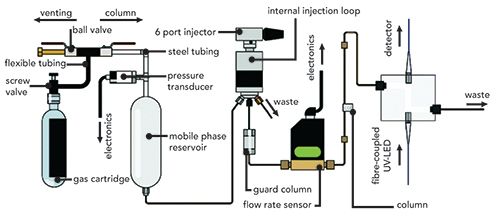
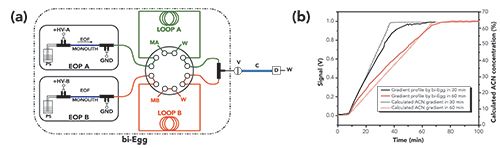
Finally, multiple groups have implemented the use of electroosmotic pumping mechanisms for the design of portable LC instruments (99–101). Electroosmotic pumps involve the application of high voltages to charged surface systems (such as fused silica walls, bare silica particles, or silica monoliths) to generate electroosmotic flow (EOF) to pump mobile phase through the column (102). Recent advances have significantly increased the demonstrated pressure limits of these pumps (up to 1200 bar) (103) and their capabilities for generating gradient mobile phase flow (Figure 7) (104,105). An integrated system was demonstrated that combined an electroosmotic pump (including the necessary high-voltage power supplies), an injection valve, and a column (100). It was coupled to an external UV absorbance detector and a MS detector for the separation of both peptides and proteins, although neither detection mode was portable with this design. A more complete system implemented electroosmotic pumping and used a microfluidic LC column with an LED-based absorbance detector and on-chip valving (101); it was applied for the measurement of glycated hemoglobin, a method used for diabetes screening that could be beneficial in resource-limited locations. The use of electroosmotic pumping has significant advantages for the design of miniaturized instruments, especially when utilizing small footprint high-voltage power supplies (106), but challenges for broad applicability remain. The flow rates are highly dependent on the surface chemistry of the EOF pump system, as well as experimental chromatographic parameters including mobile phase selection and column flow resistance. Thus, flexibility for a wide variety of separation conditions and methods is more difficult to achieve than with the syringe-based pumps described for the first two systems.
Figure 7: In panel (a), the flow diagram for a binary electroosmotic pump gradient generator (bi-Egg) used for capillary LC is shown. Pumping solution reservoir (PS), input from the high voltage supply (HV), electric ground connection (GND), mobile phase A & B reservoirs (MA, MB), gradient storage loops for each mobile phase component (LOOP A, LOOB B), waste reservoirs (W), injection valve (V), capillary LC column (C), and detector (D) are shown. Panel (b) shows the programmed (dashed line) and observed (solid line) gradient curves for 30 min (black line) and 60 min (red line) linear gradient ramps. Adapted with permission from (104).
Conclusions and Future Outlook
The increase of research activity aimed toward the development of higher efficiency capillary-scale chromatographic columns and portable capillary LC instrumentation in recent years demonstrates a push for new separation technology to solve modern problems in chemical analysis. However, without input from prospective users of such technology, it is unlikely that these columns and instruments will find broader acceptance from the greater scientific community. One of the most widely used applications of liquid chromatography is for chemical analysis within the pharmaceutical industry. The Enabling Technologies Consortium (ETC) is a group with membership composed of several major pharmaceutical manufacturers that promotes precompetitive collaborations focused on improved processes for chemical manufacturing and analysis (107). This organization has recently proposed a list of desired instrument capabilities for compact LC technology, demonstrating an interest in pursuing the integration of such instruments into pharmaceutical workflows. Given that the pharmaceutical industry is one of the most critical parts of the greater chromatographic community, their interest in portable LC instrumentation is a strong indicator of the need for such platforms. This is especially true in the area of process analytical technology (PAT), where real-time feedback based on analytical data acquired directly from the manufacturing process stream is often needed during drug production (108), although continued development in on-line sampling technology is needed to take full advantage of these smaller separation systems (109). Other application areas that will likely benefit from improvements to these portable platforms include forensics (110), point-of-care diagnostics (111), food and beverage testing (112), agricultural analysis (113), and environmental monitoring (114).
Beyond these application areas, there are a variety of instances in which portable instrumentation may be the preferred, or only, way to conduct a LC separation (115). Because of the strict focus on payload weight and volume in space vessels, extraterrestrial analysis requires the use of miniaturized platforms, especially when conducted remotely to guide additional mission tasks (116). This can also be true for difficult-to-reach areas where transportation times are a major hindrance to evaluating samples, such as pollution or commercial testing in isolated ocean sites (117). In air-sensitive environments, such as glove boxes commonly found in laboratories, analysis within the controlled area may be preferred to moving samples in and out of the box for safety and convenience (118); traditional benchtop instrumentation is typically too large to fit in these boxes. Instances where environmental hazards or other issues related to dangerous exposure may require fast analysis of unknown samples to provide information for defense or public health decision-making are also best served by portable instrumentation (119,120). Direct on-site sampling prevents potential issues in method design and analyte quantification that could result from transporting field samples to the laboratory, which suggests the need for continued progress in on-site sample preparation techniques in parallel to portable instrument design (121). Finally, the added “green” benefit of reduced solvent use that is achieved through the use of capillary-scale columns (122) is especially pertinent when there is a need to eliminate chemical waste generation in non-laboratory settings.
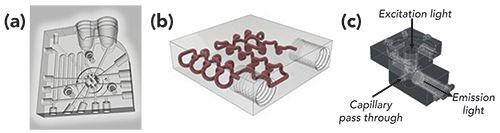
Both column and instrument design will also be impacted by manufacturing advances enabled by three-dimensional (3D) printing (123–125). Individual instrument components, including valves (126,127), fluidic connections and column platforms (128–133), and detectors (134–136) have all been fabricated using additive manufacturing techniques (Figure 8). The early stages of direct printing of stationary phase supports and column beds have also been reported (137–139), with the ultimate goal of achieving the maximum chromatographic efficiency that is theoretically possible (47,140,141). Although significant challenges to generating an ideal stationary phase support structure for analytical separations in a reasonable time and for a reasonable cost still exist, there are many opportunities for new manufacturing strategies to play a role in the future of chromatographic separations (47,142). Instrument design related to system control and data acquisition may utilize microcontrollers and single-board computers, technology that is often used to control 3D printers, to achieve reduced cost and size in portable instrumentation (143–145).
Figure 8: 3D printed designs for (a) manifold enabling in-valve sample handling and assays, (b) 3D serpentine column channel, and (c) an on-column capillary LED-induced fluorescence detector. Adapted with permission from (126,130,136).
The potential for smaller chromatographic separation columns and instruments to transform the world of chemical analysis is high. More than fifty years after the initial development of modern HPLC technology, the field has been completely transformed in terms of instrument design, column performance, and analytical throughput. The next step for many chemical measurement techniques involves taking the laboratory to the sample rather than the sample to the laboratory, an approach that can be achieved for LC by implementing many of the advances discussed here. As my research group continues our work in this area, along with the many other separation scientists pursuing the goal of better, faster, and cheaper measurement techniques, we hope to be able to say that the field has completely transformed again 50 years from now!
Acknowledgments
Edward Franklin (Regis Technologies), Justin Godinho (Advanced Materials Technology), and Kaitlin Grinias (GlaxoSmithKline) are thanked for their helpful discussions regarding these topics. Funding for the Grinias Laboratory on portable LC in collaboration with Axcend Corporation has been supported by the National Institute of Drug Abuse and the National Institute of General Medical Sciences of the National Institutes of Health under award numbers R41DA045382 and R44GM137649. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
References
- M.V. Novotny, J. Chromatogr. A 1523, 3–16 (2017).
- J.W. Jorgenson, Annu. Rev. Anal. Chem. 3(1), 129–150 (2010).
- J.E. MacNair, K.C. Lewis, and J.W. Jorgenson, Anal. Chem. 69(6), 983–989 (1997).
- J.E. MacNair, K.D. Patel, and J.W. Jorgenson, Anal. Chem.71(3), 700–708 (1999).
- L. Tolley, J.W. Jorgenson, and M.A. Moseley, Anal. Chem. 73(13), 2985–2991 (2001).
- K.D. Patel, A.D. Jerkovich, J.C. Link, and J.W. Jorgenson, Anal. Chem. 76(19), 5777–5786 (2004).
- J.S. Mellors and J.W. Jorgenson, Anal. Chem.76(18), 5441–5450 (2004).
- J.P. Grinias and G.A. Kresge, LCGC North Am. 35(8), 515–516 (2017).
- J.P. Grinias et al., LCGC North Am. 38(2), 75–80 (2020).
- L.E. Blue et al., J. Chromatogr. A 1523, 17–39 (2017).
- A.S. Kaplitz et al., Anal. Chem.92(1), 67–84 (2020).
- K. Mejía-Carmona, J. Soares da Silva Burato, J.V.B. Borsatto, A.L. de Toffoli, and F.M. Lanças, TrAC-Trends Anal. Chem.122, 115735 (2020).
- M.J. Sorensen, B.G. Anderson, and R.T. Kennedy, TrAC-Trends Anal. Chem. 124, 115810 (2020).
- S.R. Wilson, C. Olsen, and E. Lundanes, Analyst144(24), 7090–7104 (2019).
- S. Bruns et al., Anal. Chem.82(15), 6569–6575 (2010).
- D. Hlushkou, S. Bruns, A. Höltzel, and U. Tallarek, Anal. Chem.82(17), 7150–7159 (2010).
- S. Bruns and U. Tallarek, J. Chromatogr. A 1218(14), 1849–1860 (2011).
- S. Bruns, J.P. Grinias, L.E. Blue, J.W. Jorgenson, and U. Tallarek, Anal. Chem.84(10), 4496–4503 (2012).
- S. Hsieh and J.W. Jorgenson, Anal. Chem.68(7), 1212–1217 (1996).
- E.G. Franklin, “Utilization of Long Columns Packed with sub-2 μm Particles Operated at High Pressures and Elevated Temperatures for High-Efficiency One-Dimensional Liquid Chromatographic Separations” (2012).
- S. Bruns et al., J. Chromatogr. A1318, 189–197 (2013).
- A.E. Reising, J.M. Godinho, K. Hormann, J.W. Jorgenson, and U. Tallarek, J. Chromatogr. A1436, 118–132 (2016).
- A.E. Reising, J.M. Godinho, J.W. Jorgenson, and U. Tallarek, J. Chromatogr. A 1504, 71–82 (2017).
- J.M. Godinho, A.E. Reising, U. Tallarek, and J.W. Jorgenson, J. Chromatogr. A1462, 165–169 (2016).
- A.E. Reising, J.M. Godinho, J. Bernzen, J.W. Jorgenson, and U. Tallarek, J. Chromatogr. A 1569, 44–52 (2018).
- F. Gritti and M.F. Wahab, LCGC North Am.36(2), 82–98 (2018).
- M.F. Wahab, D.C. Patel, R.M. Wimalasinghe, and D.W. Armstrong, Anal. Chem. 89(16), 8177–8191 (2017).
- F. Gritti, J. Chromatogr. A1540, 55–67 (2018).
- A.E. Reising, S. Schlabach, V. Baranau, D. Stoeckel, and U. Tallarek, J. Chromatogr. A 1513, 172–182 (2017).
- F. Gritti, J. Hochstrasser, A. Svidrytski, D. Hlushkou, and U. Tallarek, J. Chromatogr. A 460991 (2020). doi:10.1016/j.chroma.2020.460991.
- S.I. Kovalchuk, O.N. Jensen, and A. Rogowska-Wrzesinska, Mol. Cell. Proteomics18(2), 383–390 (2019).
- J.P. Kutter, J. Chromatogr. A1221, 72–82 (2012).
- J.P. Grinias and R.T. Kennedy, TrAC-Trends Anal. Chem.81, 110–117 (2016).
- A. Kecskemeti and A. Gaspar, Anal. Chim. Acta 1021, 1–19 (2018).
- X. Yuan and R.D. Oleschuk, Anal. Chem.90(1), 283–301 (2018).
- S. Khirevich, A. Höltzel, D. Hlushkou, and U. Tallarek, Anal. Chem. 79(24), 9340–9349 (2007).
- S. Ehlert et al., Anal. Chem. 80(15), 5945–5950 (2008).
- S. Khirevich, A. Höltzel, D. Hlushkou, A. Seidel-Morgenstern, and U. Tallarek, Lab Chip 8(11), 1801–1808 (2008).
- S. Jung et al., Anal. Chem. 81(24), 10193–10200 (2009).
- S. Khirevich, A. Höltzel, S. Ehlert, A. Seidel-Morgenstern, and U. Tallarek, Anal. Chem. 81(12), 4937–4945 (2009).
- W. Wang et al., Anal. Chem.86(4), 1958–1964 (2014).
- S. Wang et al., Anal. Chim. Acta 1018, 70–77 (2018).
- K. Li et al., Talanta215 (2020).
- M. Vázquez and B. Paull, Anal. Chim. Acta668(2), 100–113 (2010).
- R. Knob, V. Sahore, M. Sonker, and A.T. Woolley, Biomicrofluidics 10(3) (2016).
- D. Cabooter et al., J. Chromatogr. A1325, 72–82 (2014).
- K. Broeckhoven et al., LCGC North Am. 36(6), 9–17 (2018).
- A.G. Chambers, J.S. Mellors, W.H. Henley, and J.M. Ramsey, Anal. Chem. 83(3), 842–849 (2011).
- S. Wouters et al., J. Chromatogr. A1355, 253–260 (2014).
- S. Wouters et al., J. Chromatogr. A1523, 224–233 (2017).
- J.P. Grinias and R.T. Kennedy, Chromatography 2(3), 502–514 (2015).
- C. Lotter et al., Anal. Chem.88(15), 7481–7486 (2016).
- M. Gilar et al., J. Chromatogr. A1533, 127–135 (2018).
- M. Gilar, T.S. McDonald, and F. Gritti, J. Chromatogr. A1470, 76–83 (2016).
- F. Gritti and G. Guiochon, J. Chromatogr. A1228, 2–19 (2012).
- S.C. Lam, E. Sanz Rodriguez, P.R. Haddad, and B. Paull, Analyst 144(11), 3464–3482 (2019).
- T. Hara et al., Anal. Chem. 88(20), 10158–10166 (2016).
- T. Hara et al., J. Chromatogr. A1580, 63–71 (2018).
- P. Xiang, Y. Yang, Z. Zhao, M. Chen, and S. Liu, Anal. Chem.91(16), 10738–10743 (2019).
- P. Xiang et al., Anal. Chem.92(7), 4711–4715 (2020).
- G. Desmet and S. Eeltink, Anal. Chem. 85(2), 543–556 (2013).
- W. De Malsche, J. Op De Beeck, S. De Bruyne, H. Gardeniers, and G. Desmet, Anal. Chem. 84(3), 1214–1219 (2012).
- G. Desmet, M. Callewaert, H. Ottevaere, and W. De Malsche, Anal. Chem.87(14), 7382–7388 (2015).
- S. Futagami et al., Analyst 144(5), 1809–1817 (2019).
- M. Baca, G. Desmet, H. Ottevaere, and W. De Malsche, Anal. Chem.91(17), 10932–10936 (2019).
- K. Sandra et al., LCGC North Am.30(6s), 6–13 (2017).
- G. Nys, G. Cobraiville, and M. Fillet, Anal. Chim. Acta 1086, 1–13 (2019).
- J. Stadlmann et al., Anal. Chem.91(22), 14203–14207 (2019).
- G. Tóth, T. PaniÄ-JankoviÄ, and G. MituloviÄ, J. Chromatogr. A1603, 426–432 (2019).
- T. Hara, S. Futagami, W. De Malsche, G.V. Baron, and G. Desmet, J. Chromatogr. A 1552, 87–91 (2018).
- S. Futagami et al., J. Chromatogr. A 1595, 58–65 (2019).
- D.T. Snyder, C.J. Pulliam, Z. Ouyang, and R.G. Cooks, Anal. Chem.88(1), 2–29 (2016).
- X. Xu, L. Li, and S.G. Weber, TrAC-Trends Anal. Chem.26(1), 68–79 (2007).
- M. Macka, T. Piasecki, and P.K. Dasgupta, Annu. Rev. Anal. Chem. 7(1), 183–207 (2014).
- M. Zhang, S.C. Phung, P. Smejkal, R.M. Guijt, and M.C. Breadmore, Trends Environ. Anal. Chem.18, 1–10 (2018).
- C.F. Poole, in Liquid Chromatography Applications, S. Fanali, P.R. Haddad, C.F. Poole, and M-L. Riekkola, Eds. (Elsevier, Amsterdam, Netherlands, 2nd Ed., 2017), pp. 39–68.
- E. Vasconcelos Soares Maciel, A.L. de Toffoli, E. Sobieski, C.E. Domingues Nazário, and F.M. Lanças, Anal. Chim. Acta 1103, 11–31 (2020).
- K.B. Lynch, A. Chen, and S. Liu, Talanta177, 94–103 (2018).
- G. Desmet and K. Broeckhoven, TrAC Trends Anal. Chem. 119, 115619 (2019).
- J.P. Grinias, B. Bunner, M. Gilar, and J.W. Jorgenson, Chromatography2(4), 669–690 (2015).
- E.G. Franklin and T.W. Kahler, LCGC North Am.34(6), 388–399 (2016).
- S. Sharma et al., J. Chromatogr. A1421, 38–47 (2015).
- S.W. Foster et al., J. Sep. Sci. 43, 1623–1627 (2020).
- S. Sharma et al., J. Chromatogr. A1327, 80–89 (2014).
- S. Sharma et al., Anal. Chem.87(20), 10457–10461 (2015).
- S. Sharma, H.D. Tolley, P.B. Farnsworth, and M.L. Lee, Anal. Chem.87(2), 1381–1386 (2015).
- X. Xie et al., J. Chromatogr. A1523, 242–247 (2017).
- X. Zhao et al., Anal. Chem.89(1), 807–812 (2017).
- X. Xie, L. Patil, and M. Lee, “Utilizing Portable Capillary LC for Automated On-line Process and Reaction Monitoring” at Pittcon 2020 (Chicago, IL, 2020).
- M. Lee et al., “Breadth of Applications of Portable Capillary Liquid Chromatography” at Pittcon 2020 (Chicago, IL, 2020).
- J.P. Grinias, S. Foster, M. Pham, and G. Kresge, “The Potential for Portable Liquid Chromatography in Chemical Analysis” at Pittcon 2020 (Chicago, IL, 2020).
- S.C. Lam et al., Anal. Chim. Acta 1101â, 199–210 (2020).
- Y. Li et al., Anal. Chim. Acta896, 166–176 (2015).
- E. Murray et al., J. Sep. Sci.41(16), 3224–3231 (2018).
- Y. Li et al., Talanta180, 32–35 (2018).
- S. C. Lam, V. Gupta, P. R. Haddad, and B. Paull, Anal. Chem. 91(14), 8795–8800 (2019).
- Y. Li, P.N. Nesterenko, R. Stanley, B. Paull, and M. Macka, Anal. Chim. Acta1032, 197–202 (2018).
- S. Chatzimichail, D. Casey, and A. Salehi-Reyhani, Analyst144(21), 6207–6213 (2019).
- A. Ishida et al., Anal. Sci.31(11), 1163–1169 (2015).
- K.B. Lynch, A. Chen, Y. Yang, J.J. Lu, and S. Liu, J. Sep. Sci.40(13), 2752–2758 (2017).
- D. Li et al., Sensors Actuators, B Chem. 305, 127484 (2020).
- L. Li, X. Wang, Q. Pu, and S. Liu, Anal. Chim. Acta1060, 1–16 (2019).
- C. Gu et al., Anal. Chem.84(21), 9609–9614 (2012).
- 1L. Zhou, J.J. Lu, C. Gu, and S. Liu, Anal. Chem.86(24), 12214–12219 (2014).
- A. Chen et al., Anal. Chim. Acta887, 230–236 (2015).
- P. KubáÅ, F. Foret, and G. Erny, Electrophoresis 40(1), 65–78 (2019).
- C.J. Welch et al., Org. Process Res. Dev.21(3), 414–419 (2017).
- A. Chanda et al., Org. Process Res. Dev.19(1), 63–83 (2015).
- C.A. Challener, Pharm. Technol.41(4), 22–27 (2017).
- W.R. de Araujo et al., Anal. Chim. Acta1034, 1–21 (2018).
- M. Zarei, TrAC-Trends Anal. Chem. 91, 26–41 (2017).
- G. Rateni, P. Dario, and F. Cavallo, Sensors17(6), 1453 (2017).
- A. Cassedy, E. Mullins, and R. O’Kennedy, Biotechnol. Adv.39 (2020).
- R. Pol, F. Céspedes, D. Gabriel, and M. Baeza, TrAC-Trends Anal. Chem.95, 62–68 (2017).
- M. Kaljurand, Trends Environ. Anal. Chem.1 (2014).
- C.P. Shelor et al., Astrobiology 14(7), 577–588 (2014).
- J. Birch, Pure Appl. Chem.90(10), 1625–1630 (2018).
- D.J. Leggett, Process Saf. Prog. 31(4), 393–397 (2012).
- I.S. Che Sulaiman et al., Forensic Toxicol. Dec. 16 (2019). doi:10.1007/s11419-019-00513-x.
- R. Jackson and B.A. Logue, Anal. Chim. Acta960, 18–39 (2017).
- J. Pawliszyn, TrAC-Trends Anal. Chem.25(7), 633–634 (2006).
- J. PÅotka et al., J. Chromatogr. A1307, 1–20 (2013).
- U. Kalsoom, P. N. Nesterenko, and B. Paull, TrAC-Trends Anal. Chem.105, 492–502 (2018).
- B. Paull, LCGC Europe 30(11), 611–612 (2017).
- S. Dimartino, LCGC Europe32(6), 317–318 (2019).
- D.J. Cocovi-Solberg, M. Rosende, M. Michalec, and M. Miró, Anal. Chem.91(1), 1140–1149 (2019).
- S.H. Nawada, T. Aalbers, and P.J. Schoenmakers, Chem. Eng. Sci.207, 1040–1048 (2019).
- S. Sandron et al., Analyst139(24), 6343–6347 (2014).
- V. Gupta et al., Anal. Chim. Acta 910, 84–94 (2016).
- V. Gupta, S. Beirne, P. N. Nesterenko, and B. Paull, Anal. Chem.90(2), 1186–1194 (2018).
- T. Adamopoulou, S. Deridder, G. Desmet, and P.J. Schoenmakers, J. Chromatogr. A 1577, 120–123 (2018).
- M. Komendová, S. Nawada, R. Metelka, P.J. Schoenmakers, and J. Urban, J. Chromatogr. A1610 (2020).
- M. Passamonti et al., Anal. Chem. 92(3), 2589–2596 (2020).
- J. Prikryl and F. Foret, Anal. Chem.86(24), 11951–11956 (2014).
- F. Cecil et al., Anal. Chim. Acta965, 131–136 (2017).
- L.D. Casto, K.B. Do, and C.A. Baker, Anal. Chem. 91(15), 9451–9457 (2019).
- R.J. Vonk, A. Vaast, S. Eeltink, and P.J. Schoenmakers, J. Chromatogr. A 1359, 162–169 (2014).
- C. Fee, S. Nawada, and S. Dimartino, J. Chromatogr. A1333, 18–24 (2014).
- S. Nawada, S. Dimartino, and C. Fee, Chem. Eng. Sci. 164, 90–98 (2017).
- C. Salmean and S. Dimartino, Chromatographia82(1), 443–463 (2019).
- F. Dolamore, S. Dimartino, and C. J. Fee, Anal. Chem.91(23), 15009–15016 (2019).
- G. Desmet, “Hot Topics in HPLC Part II: The Possibilities and Challenges of Producing Chromatographic Media with Two-Photon Polymerization Printing LC–GC” (2020). at <http://www.chromatographyonline.com/hot-topics-hplc-part-ii>.
- J.P. Grinias, J.T. Whitfield, E.D. Guetschow, and R.T. Kennedy, J. Chem. Educ.93(7), 1316–1319 (2016).
- S.W. Foster, M.J. Alirangues, J.A. Naese, E. Constans, and J.P. Grinias, J. Chromatogr. A1603, 396–400 (2019).
- P.L. Urban, Angew. Chemie - Int. Ed. 57(34), 11074–11077 (2018).
James P. Grinias is an assistant professor in the Department of Chemistry & Biochemistry at Rowan University, in Glassboro, New Jersey. Direct correspondence to: grinias@rowan.edu

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.

![[1]L.jpg [1]L.jpg](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2F0vv8moc6%2Fchroma%2F2ca2ff2694c34a129d7ae348d24f6041f6ff9fdf-500x129.jpg%3Ffit%3Dcrop%26auto%3Dformat&w=3840&q=75)


![[1]L1593120170948.jpg [1]L1593120170948.jpg](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2F0vv8moc6%2Fchroma%2F77f8f859bb7a2582ccded7f5dd0b4cc0efde35c1-500x137.jpg%3Ffit%3Dcrop%26auto%3Dformat&w=3840&q=75)

