PFAS Analytical Challenges: Regulations and Methods Unravelled
Per- and polyfluorinated alkyl substances (PFAS) are manufactured chemicals that have been used in a wide variety of common consumer products and industrial activities. Known as “forever chemicals” because they do not breakdown and can bioaccumulate, their impact to the environment and human health has been under the microscope in recent months. PFAS have made their way into public drinking water supplies, with estimates that over 200 million Americans have tap water that is contaminated. Testing for PFAS is an incredible challenge and laboratories must be ready to meet demands for sample throughput, trace detection, and complex matrices, all while keeping pace with regulations. By utilizing the sensitivity and specificity of liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS), analytical chemists can successfully identify and measure PFAS and adapt to the evolving regulatory requirements for a wide variety of sample matrices.
Per- and polyfluorinated alkyl substances (PFAS) are manufactured chemicals that have been used in a variety of common consumer products since the 1940s. For example, they keep food from sticking to cookware and packaging, they make carpets stain-resistant and fabrics waterproof, and they are used to create highly effective firefighting foams. The widespread use of these chemicals has resulted in deposition into the environment, where they have eventually found their way into public drinking water and food supplies. This broad category of chemicals is highly suspected of having adverse health impacts, such as immune suppression, liver damage, thyroid disease, increased cholesterol, and cancer. Because of the strength of the carbon‑fluorine bond, PFAS compounds do not readily degrade and are persistent in the environment, earning them the nickname “forever chemicals”.
What are these Chemicals and How Widespread is the Impact?
There is no specific or universally accepted definition of what determines a chemical to be categorized as a PFAS moiety. It is generally recognized to be a chemical that has a carbon-fluorine hydrocarbon chain attached to a functional group. Examples are carboxylic, sulfonic, and phosphinic acids, or ethers, sulphates, and phosphates. Early interest in PFAS chemicals was traditionally on two prevalent compounds: perfluorooctanoic acid (PFOA), primarily associated with the manufacture of non‑stick coatings, and perfluorooctanesulfonic acid (PFOS), a main component in firefighting foams. These compounds have hydrocarbon chains that are fully fluorinated, meaning all hydrogens are substituted by a fluorine, and are referred to as PFCs (perfluoro‑chemicals).
As scientists have continued to explore where and how fluorochemicals have been or are currently used, the family of PFAS chemicals that are of interest has grown significantly and now includes per- and polyfluorinated versions of many classes of hydrocarbons. The US Environmental Protection Agency (EPA) Master List of PFAS Substances includes over 5000 chemicals whose structures have been defined, and research continues to elucidate even more with powerful analytical techniques.
Grass roots efforts to educate communities on environmental releases and impacts has meant that public awareness is high regarding human exposure, and communities and environmental groups are pressing for the removal of these contaminants from their drinking water and food supplies. Recent reports estimate there are over 2800 water sources contaminated with PFAS throughout 50 states in the US, affecting 200 million people (1).
US Regulations at the Federal and State Level
Along with many countries around the world, the United States are putting in place the regulatory framework needed to protect the environment and populations from exposure to these harmful chemicals.
The EPA established the PFAS Action Plan in early 2019 in response to growing public concern. According to the EPA, “This Action Plan describes the EPA’s approach to identifying and understanding PFAS, approaches to addressing current PFAS contamination, preventing future contamination, and effectively communicating with the public about PFAS”(2). In October 2021, EPA released their PFAS Strategic Roadmap, outlining a commitment to actions focused on the research, restriction, and remediation of PFAS (3).
While significant progress has been made towards understanding the universe of these chemicals, and techniques to remediate or remove them are improving, there are no federal regulations yet established for protecting drinking water sources and supplies, or other environmental matrices. Although the EPA established a health advisory level of 70 parts per trillion (ppt) for PFOS, PFOA, or a combination of the two chemicals in drinking water, as an advisory guideline, there is no enforcement or monitoring mandate.
To fill this gap, many US states that have known releases of these chemicals impacting public and private drinking water sources have taken the lead on establishing regulations to protect their water supplies and provide a legal framework for enforcing cleanup. Under the Safe Drinking Water Act, individual states can implement their own regulations as long as they meet or exceed federal standards, and this is happening in lieu of federal action. Currently, 38 states have policies in place for addressing PFAS in the environment, nine have established legally enforceable maximum contaminant levels (MCLs) for PFAS chemicals in drinking water, and MCL regulations are in progress in four more. Many of these regulations include compounds used as replacements for PFOA or PFOS, such as hexafluoropropylene oxide dimer acid (GenX), and shorter chain variations, and are set as low as 6 ppt (Michigan) or 13 ppt (New Jersey), for example.
Some states, like Maine, have banned PFAS chemicals from use in consumer products, with other states considering putting a similar ban into law.
Global Perspective
As in the United States, regulations are being driven by growing public awareness of sites where these chemicals have been released and are now impacting water sources, food supplies, and public health. Some countries are moving faster than others. For example, the European Union, Canada, and Australia have all begun tightening controls over these chemicals, establishing regulations for water, food, and food packaging materials, potentially banning the use of these chemicals, and initiating management plans and further studies to respond to the growing evidence of health impacts.
In Asia, research on use and impact is ongoing but regulations are lacking. Many countries are part of the International Pollutants Elimination Network (IPEN), however, and this organization provided a comprehensive report in 2019 highlighting concerns about the prevalence of substances in Asia (4).
Testing Methods: A Variety of Solutions Using LC–MS/MS (5)
As research continues and regulations develop around PFAS, there is an increasing need for robust, reliable methods that support the detection of these chemicals in a wide variety of matrices at ultralow concentrations. Liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) is the ideal analytical solution for delivering high sensitivity and analyte specificity for these types of chemicals. Tandem MS technology is an uncomplicated platform that is easy to operate and designed for demanding quantitative applications. Not only are chromatographic interferences common to environmental samples reduced with multiple reaction monitoring (MRM), this technology also has wide dynamic ranges that can handle high concentration samples.
Agencies like ASTM (American Society for Testing and Materials) and ISO (International Organization for Standardization), along with the EPA, have utilized the sensitivity and robustness of tandem quadrupole mass spectrometry technology to develop methods that can analyze a wide range of complex matrices, and that today cover upwards of 40 or more PFAS that have been identified as being significant for monitoring due to known release, use, or toxicity.
Early methods focused on legacy, longer chain PFAS for drinking water matrices only. EPA method 537 (6) was published in 2009 for testing drinking water for 14 compounds. Testing demands, however, quickly expanded beyond that scope, with researchers and laboratories being challenged to test more complicated matrices (wastewater, soil, landfill leachates, biota), for an increasing variety of new and emerging PFAS chemicals, and to reach ultralow detection limits of 1 ppt or less. In response to the evolving science, the EPA developed additional, more comprehensive methods, acknowledging the need to test for emerging PFAS chemicals and for workflows to handle a wide range of environmental matrices.
Because monitoring water sources for PFAS contamination is vital for ensuring communities have access to clean water, EPA focused on drinking water testing and released method 533 in 2019 (7). It is a prescriptive testing protocol for an expanded group of PFAS chemicals (25) in public drinking water sources. By August 2021, in collaboration with the Department of Defense, EPA released method draft 1633 (8), a method designed to test for 40 individual PFAS compounds in a full suite of environmental media, including all non-potable water sources, soil, sediment, landfill leachates, biosolids, and fish tissue samples. Both methods employ a sample enrichment step as part of the sample preparation to achieve ultralow level detections—typically concentrating 250 mL of water into a 1 mL aliquot prior to analysis. A weak anion exchange resin (WAX) must be used as the solid-phase extraction media to fully recover the extended list of long- and shorter chain analytes of various chemistries.
Isotope dilution is incorporated into these methods as a strategy for increasing the accuracy of quantitation in samples that have coextracted organics or other materials that may interfere with or suppress the detection of the target compounds. Known amounts of isotopically enriched PFAS substances are added to the original sample (usually C13 substituted), and carried through extraction and analysis, where their recovery can then be measured and used to correct the parent compound for any matrix suppression that may have occurred in that specific sample.
EPA methods are required by regulatory programmes, such as the Safe Drinking Water Act and the Department of Defense for military site investigations, and are widely implemented and in use today. While they produce reliable results for many types of PFAS in a variety of matrices, they involve some complicated sample preparation and data handling steps that may not be needed for all PFAS testing programmes, and may be more costly or difficult for some laboratories to implement.
As an alternative, in December 2021, ASTM announced they will be publishing a new simple, efficient, high‑throughput method for testing non-potable water samples for PFAS, coded ASTM-D8421 (9). The method builds on earlier work published as ASTM-D7979, which covered 24 legacy PFAS. Now 44 emerging plus legacy PFAS chemicals of concern can be successfully analyzed with ASTM-D8421. Because ultrahigh-pressure liquid chromatography (UHPLC)–MS/MS platforms are exceptionally sensitive, co‑solvation direct injection of samples can be performed, eliminating complicated solid-phase extraction with no compromise in meeting trace-level detection of these chemicals. This method provides laboratories with a cost-effective means for supporting high-throughput testing of water samples in support of investigative work assessing, remediating, and monitoring water supplies and sources for PFAS contamination.
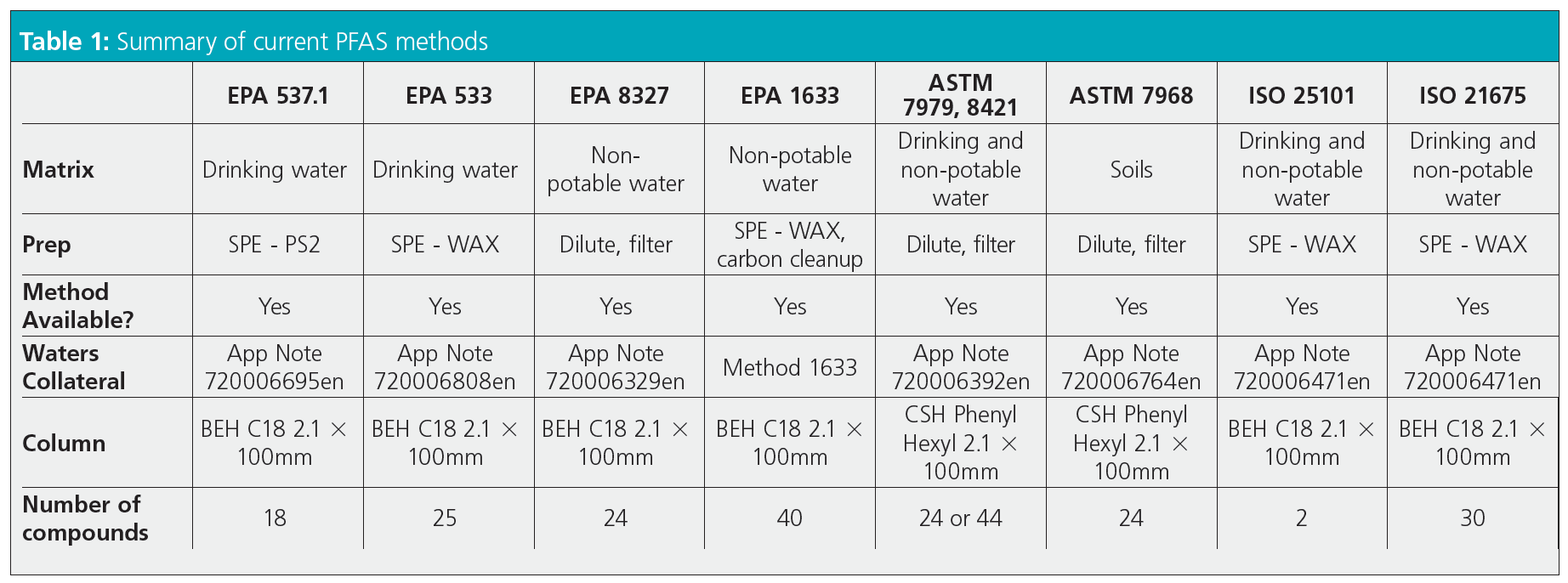
Table 1 provides a quick summary of available methods.
Emerging and Novel PFAS Discovery Through Nontarget Screening
Traditionally, environmental analytical workflows have been designed to test for a defined list of chemicals or target analytes. Once a chemical release or exposure has been brought to light, methods are set up to test for these specific chemicals. Today, researchers and environmental scientists working to gain a fuller understanding of potential human exposure to chemical compounds can utilize high resolution mass spectrometry (HRMS) tools to proactively search for these yet undiscovered chemicals in environmental, residential, and biological media.
Chemical and toxicological information pertaining to the newer formulated PFAS products is often not widely available, therefore, the use of analytical methods that can provide data on nontargeted analytes as well as targeted analytes is beneficial and can help to fully assess the environmental distribution of this class of compounds. HRMS coupled to UHPLC can be used to elucidate novel PFAS compounds. Software libraries consisting of accurate masses of molecular ions, fragment ions, isotope patterns, and retention times are continually being updated, and are available to begin assigning putative identifications to the components detected. Further discovery of PFAS not present in such libraries can be achieved by automatic searching of external databases, aided with additional MS details including common fragments, neutral loss, and mass defect searching (10).
PFAS are Everywhere
Background contamination from materials in laboratories testing for PFAS is of particular concern given that the limits of detection are so low. PFAS may be found at trace-levels as impurities in a variety of products and commonly used laboratory items, including any polytetrafluoroethylene (PTFE) products or materials (bottles, caps, solvent lines), aluminium foil, permanent markers, and methanol as just a few examples. Laboratories must take care to reduce or eliminate sources of background PFAS to ensure the accuracy of their results.
UHPLC systems can also be a source of background interference and should be outfitted with a PFAS kit to reduce or remove any background PFAS attributed to PTFE components. Mobile phase tubing should be PEEK tubing to eliminate this as a source. Additionally, since other PTFE-coated pieces inside the LC system cannot be removed, an isolator column in combination with a stainless-steel solvent delay coil can be installed after the solvent mixer. This delays the elution of any PFAS contaminants coming from the LC system or mobile phase, enough to provide chromatographic resolution between the presumptive PFAS peaks in samples and the residual background.
References
- Environmental Working Group, Interactive map: https://www.ewg.org/interactive-maps/pfas_contamination/
- USEPA, Per- and Polyfluoroalkyl Substances (PFAS) www.epa.gov/pfas. “EPA’s Per- and Polyfluoroalkyl Substances (PFAS) Action Plan” (February 2019).
- USEPA, Per- and Polyfluoroalkyl Substances (PFAS) www.epa.gov/pfas. “PFAS Strategic Roadmap: EPA’s Commitments to Action 2021-2024”, (October 2021).
- IPEN, PFAS Pollution across the Middle East and Asia: https://ipen.org/sites/default/files/documents/pfas_pollution_across_the_middle_east_and_asia.pdf
- Interstate Technology and Regulatory Council (ITRC), PFAS Technical and Regulatory Guidance Document: pfas-1.itrcweb.org
- EPA Method 537, Rev 1.1, “Determination of Selected Perfluorinated Alkyl Acids in Drinking Water by Solid Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)” (USEPA, September 2009).
- EPA Method 533, “Determination of Per- and Polyfluoroalkyl Substances in Drinking Water by Isotope Dilution Anion Exchange Solid Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry” (USEPA, November 2019).
- EPA Draft Method 1633, “Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/MS” (USEPA, August 2021).
- ASTM D8421, “Standard Test Method for Determination of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous Matrices by Co-solvation followed by Liquid Chromatography Tandem Mass Spectrometry (LC/MS/MS)” (November 2021).
- M. Twohig, G. Fujimoto, A. Mohan, K. Organtini, K. Rosnack, and S. Hird, “Approaches to Non-targeted Analyses of Per- and Polyfluoroalkyl Substances (PFAS) in Environmental Samples” Waters Corp Application Note 720007184en.
Jenifer Lewis is Senior Environmental Market Development Manager at Waters Corporation.
E-mail: Jenifer_Lewis@waters.com
Website: www.waters.com/pfas

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
















