Determination of PFAS in Water According to EU 2020/2184 and DIN 38407-42 Using Online SPE–LC–MS/MS
The EU Drinking Water Directive (EU 2020/2184) has set a limit of 0.1 µg/L for the sum of 20 per- and polyfluoroalkyl substances (PFAS) deemed of highest concern. In the work described here, the listed perfluorinated carbonic and sulfonic acids were determined by an automated method based on solid-phase extraction (SPE) with weak anion exchange sorbent combined with liquid chromatography tandem mass spectrometry (LC–MS/MS). Comprehensive rinse cycles with effluent recovery ensured inclusion of surface adsorbed analytes while minimizing sample-to-sample carryover. The required quantification limit of 1.5 ng/L was reached for all listed analytes and method accuracy was demonstrated based on analysis of spiked tap water and surface water samples.
Per- and polyfluoroalkyl substances (PFAS) are a family of highly fluorinated anthropogenic chemicals with special physicochemical properties that make them oil- and water‑repellant as well as heat-resistant. This makes them suited for many household and industrial applications. PFAS have been incorporated into commercial products such as nonstick cookware, food packaging, carpeting, cleaning products, and firefighting foams. Unfortunately, PFAS are both soluble in water and persistent, helping them to spread globally. PFAS are found not only in the environment but also in food and animal feed, in humans, and in wildlife.
PFAS are toxic, posing a threat to humans and to the environment. Among many other precautions, our drinking water must be monitored for their presence and the new EU Drinking Water Directive (EU 2020/2184) (1) includes maximum limits for total PFAS of 0.5 µg/L. For the sum of 20 PFAS of most concern the maximum limit is 0.1 µg/L, which requires a limit of detection (LOD) of 30 ng/L for the sum and 1.5 ng/L for individual compounds. Environmentally relevant PFAS should be determined not only in clean drinking water but also in ground-, surface-, and wastewater, which means that interfering compounds and matrix will need to be separated. One technique that can be used to meet these requirements is solid‑phase extraction (SPE), which is used in the DIN 38407-42 method (2). Due to the anionic properties of the analytes to be determined, weak anion exchange (WAX) resins are used. Following analyte concentration, the SPE cartridges must be very efficiently rinsed with solvent. The elution is then performed using a solution of ammonia in methanol.
Unlike traditional SPE, online SPE relies on smaller cartridges inserted into the eluent flow path that can be eluted directly onto the high performance liquid chromatography (HPLC) column. This enables quantitative transfer of analytes to the analysis system, resulting in improved limits of detection and quantitation even when sample volumes are significantly reduced.
Given how widespread PFAS are, it can be challenging to reach the required low background levels when performing the analysis. All glassware and caps must be ultra clean and only solvents and reagents of high purity used. In addition, PFAS are readily adsorbed on surfaces.
For the method described in this article, the sampler performs comprehensive rinse cycles of the vials and syringe after the sample has been injected to ensure recovery of PFAS adsorbed on the sample vial and syringe walls. The rinse effluent is injected into the system after each cycle for the highest possible recovery.
All steps of a typical SPE workflow for this method are performed automatically including conditioning, loading, rinsing, and eluting the cartridge. Following the elution step, the cartridge is removed from the HPLC mobile phase flow path, freeing the system to prepare the next sample during the ongoing LC–tandem mass spectrometry (MS/MS) analysis.
Experimental
The automated system consisted of a MultiPurpose Sampler (MPS robotic, Gerstel) and an online-SPE system (SPExos, Gerstel) coupled to an LC–MS/MS system (1260 Infinity II LC and Ultivo Triple Quadrupole LC–MS, Agilent Technologies). Weak anion exchange cartridges were used (SPExos Polymer WAX, 25–35 µm, Spark Holland). SPE elution was performed using 0.25% ammonia in methanol delivered from an additional isocratic HPLC pump. The eluate was merged with the starting level buffer of the binary analytical pump prior to it reaching the analytical column (3.0 × 100 mm, 2.7-µm Poroshell 120 EC-C18, [Agilent Technologies]). For this, a valve fitted with a special T-rotor was used in the SPExos system. Between the binary pump and MPS, a delay column (4.6 × 50 mm, 2.7-µm Poroshell 120 EC‑C18 [Agilent Technologies]) was installed.
One millilitre water samples were mixed with 50 µL internal standard solution in conical 1.1-mL vials and placed in the autosampler. Injection was performed with a 2.5 mL syringe placed into the injection valve on the MPS, fitted with a 1 mL sample loop. Prior to this, the automated workflow started out with conditioning of the cartridge, first using 0.25% ammonia in methanol, then methanol, and finally water. These steps were performed by the high-pressure dispenser (HPD) unit of the SPExos. The sample was then loaded with water and the cartridge washed with water, a rinse solution (50:50:1 acetone–acetonitrile–formic acid), and methanol. This organic wash was possible because the anionic analytes were retained by the anion exchange sorbent. A further benefit of using the sorbent was that the sample vial could be rinsed with methanol to recover adsorbed PFAS from the vial and syringe walls and the rinse effluent then added to the SPE cartridge for analysis. Methanol from a solvent reservoir on the MPS was added to the vial and the vial contents were then aspirated and injected into the sample loop on the injection valve, before being transferred to the SPE cartridge.
Finally, the online-SPE system started the HPLC pumps, which perform the SPE elution and the chromatography. Analytes were separated during a 15-min gradient run with a flow of 0.6 mL/min, employing water with 0.1% formic acid and methanol with 0.25% ammonia and 0.05% formic acid. Detection was made in dynamic multiple reaction monitoring mode (dMRM). For each target compound and isotope labelled internal standard (ISTD), two MRM transitions were chosen—one quantifier and one qualifier (except PFBA and PFPeA, for which only one transition had sufficient intensity).
Results and Discussion
Usually in online-SPE the elution is made with the gradient delivered by the analytical pump. However, the WAX cartridges are eluted with ammonia in methanol and this eluate cannot be transferred directly to the HPLC column. For this reason, an extra (isocratic) HPLC pump for cartridge elution was added. Subsequently, the eluate was merged with the starting level buffer of the binary analytical mobile phase. This takes place in the SPE system using a valve fitted with a special rotor. The result was a reduced methanol–water ratio and a lowered pH value, leading to analyte focusing at the beginning of the column.
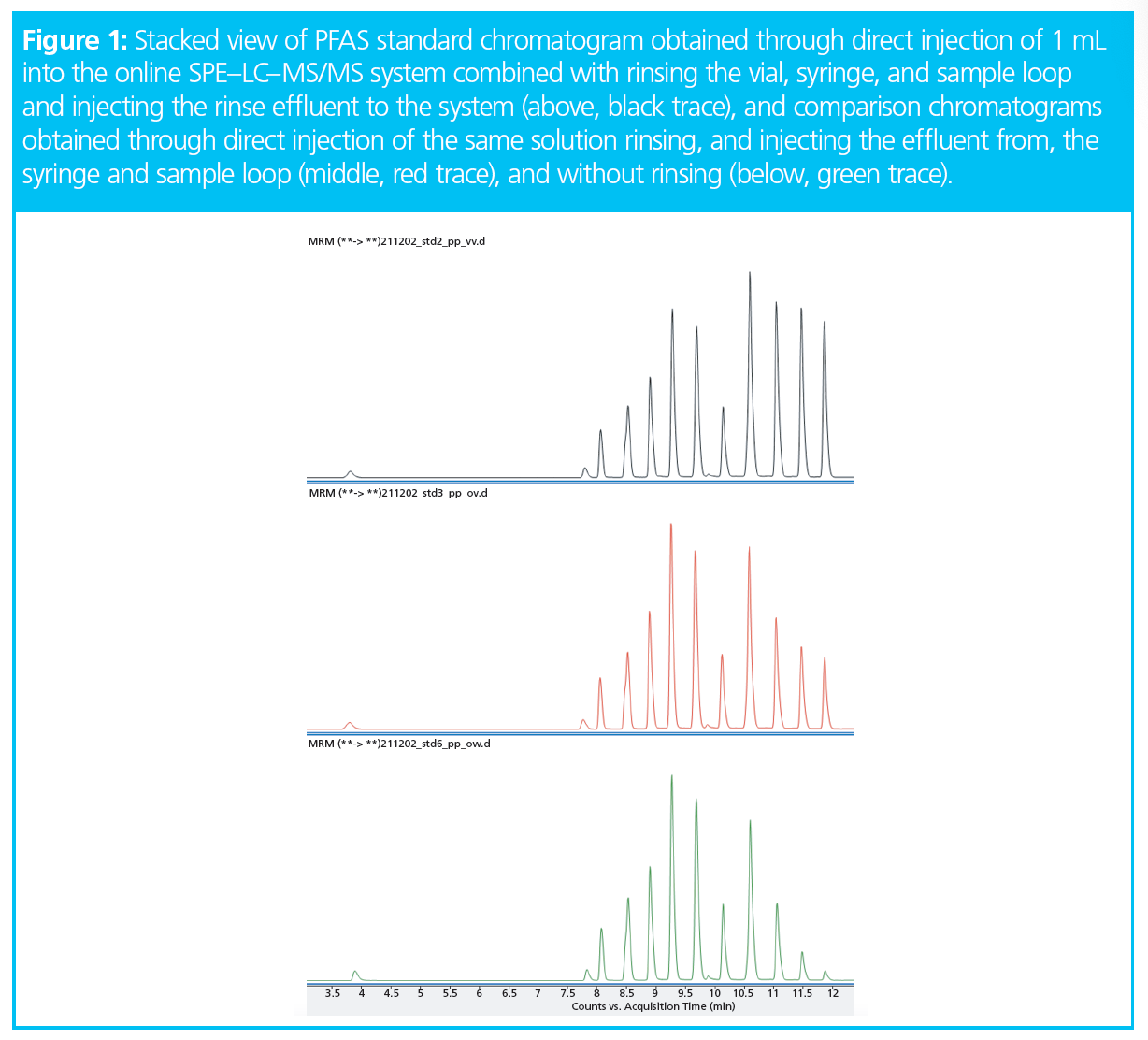
Long-chain perfluorinated acids tend to be adsorbed on surfaces. The DIN method therefore recommends solutions with at least 40% methanol to avoid loss. If samples with such high methanol content are injected onto an SPE cartridge, short‑chain PFAS are not retained. In the system used here, the sample vial, injection syringe, sample loop, and associated tubing can all be rinsed and the adsorbed analytes released, recovered, and transferred to the SPE cartridge. The effect of this can be seen in Figure 1, in which a standard chromatogram obtained using this procedure (black trace) is compared to chromatograms of the same standard obtained rinsing, and injecting the effluent from, only the syringe and sample loop (red trace), and completely without rinsing (green trace).
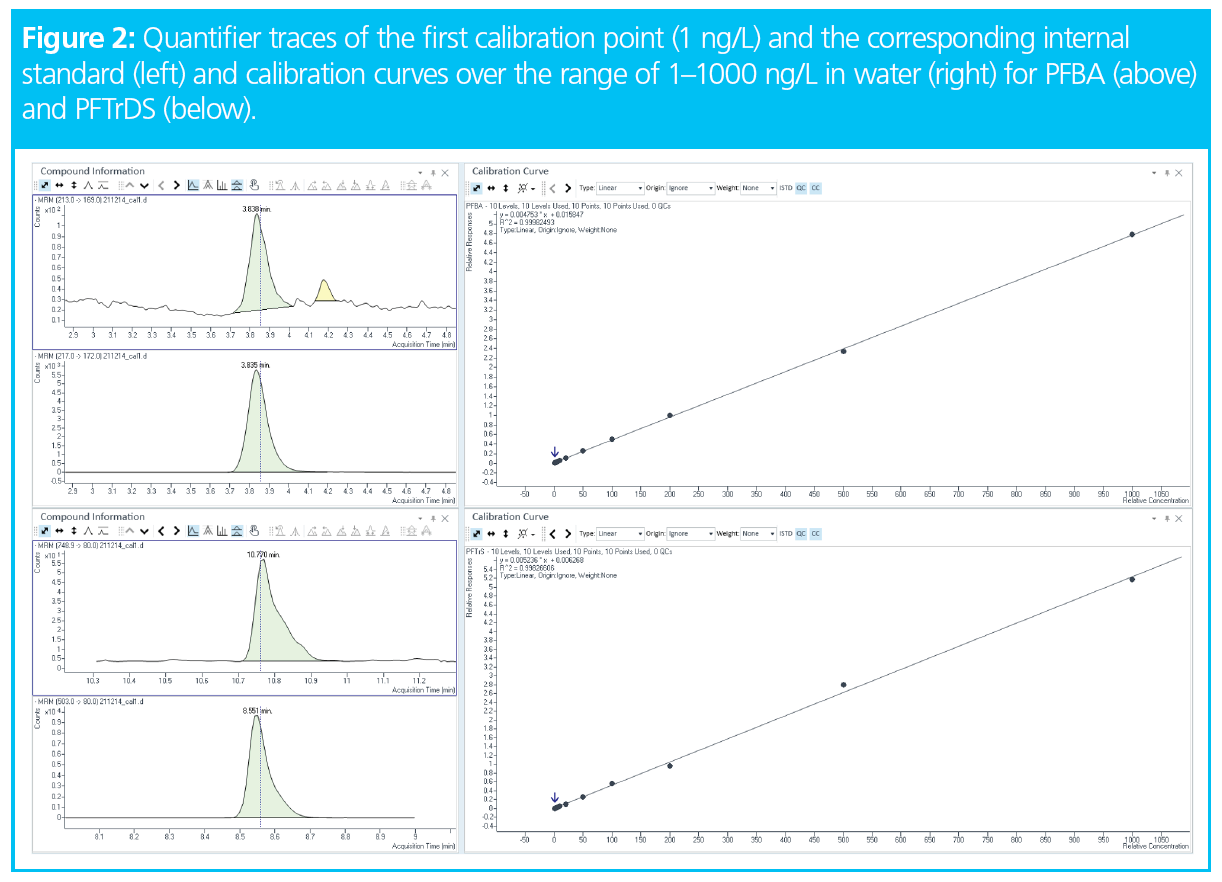
Calibration was made with pure water spiked with a solution containing all 20 PFAS (carboxylic and sulfonic acids from C4 to C13) in the range of 1–1000 ng/L. A mix of mass labelled internal standards was added to each reference solution and sample. All calibration curves were linear in this range, with R2 > 0.998. To illustrate this, curves for the first and last eluting compounds (PFBA and PFTrDS) are shown in Figure 2, together with the mass traces of the first calibration point (1 ng/L) and the corresponding internal standard.
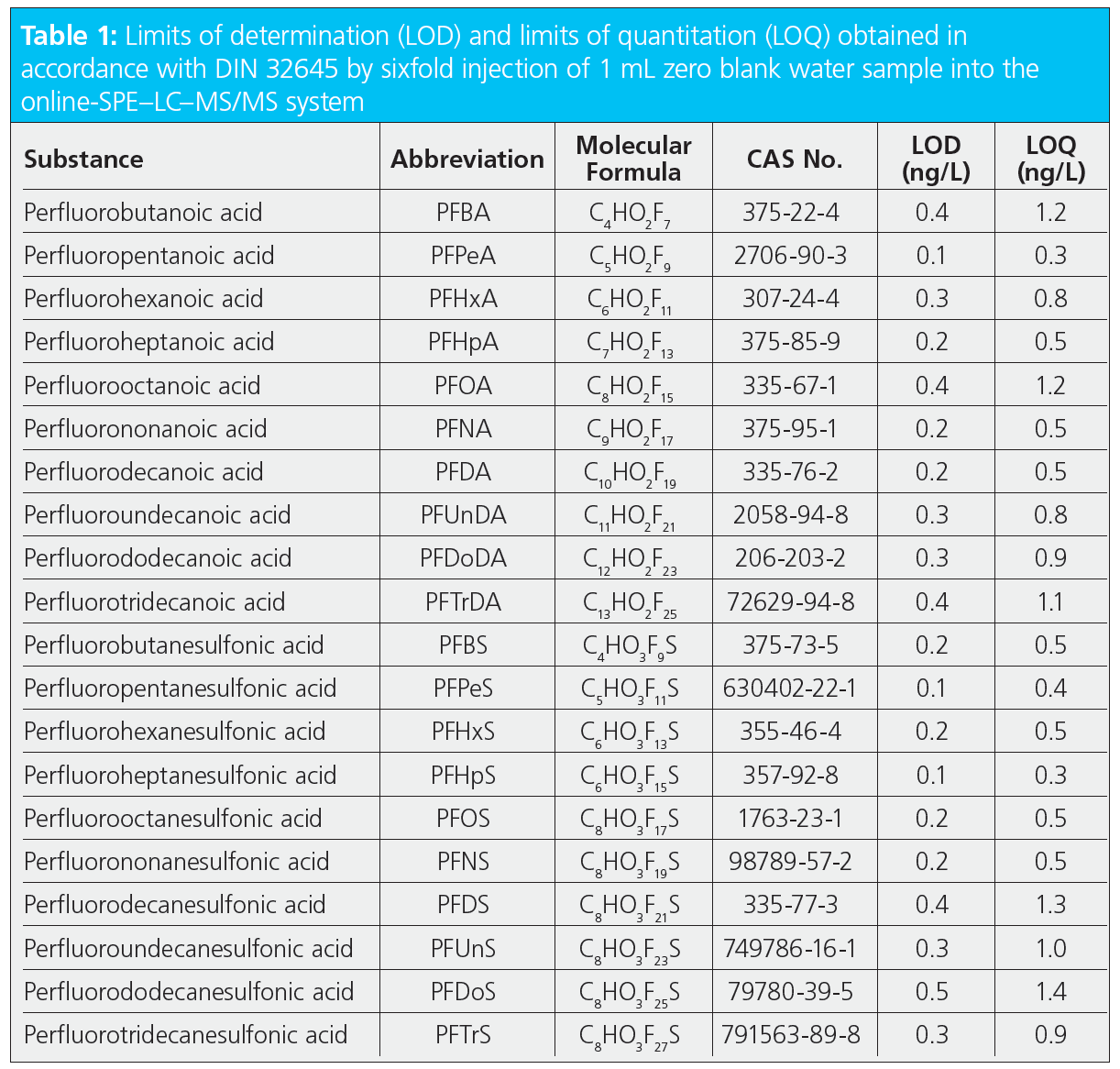
Method detection limits are not just determined by the sensitivity of the instrument but also very much by the unavoidable blank values at sub ng/L level. The statistical evaluation of repeated blank injections allowed the calculation of detection and quantification limits according to DIN 32645. The sixfold determination of blank water with the online-SPE system resulted in the quantification limits given in Table 1. They were all below 1.5 ng/L, enabling monitoring at or below the 0.1 µg/L limit for the sum of PFAS compounds as stipulated by the drinking water directive.
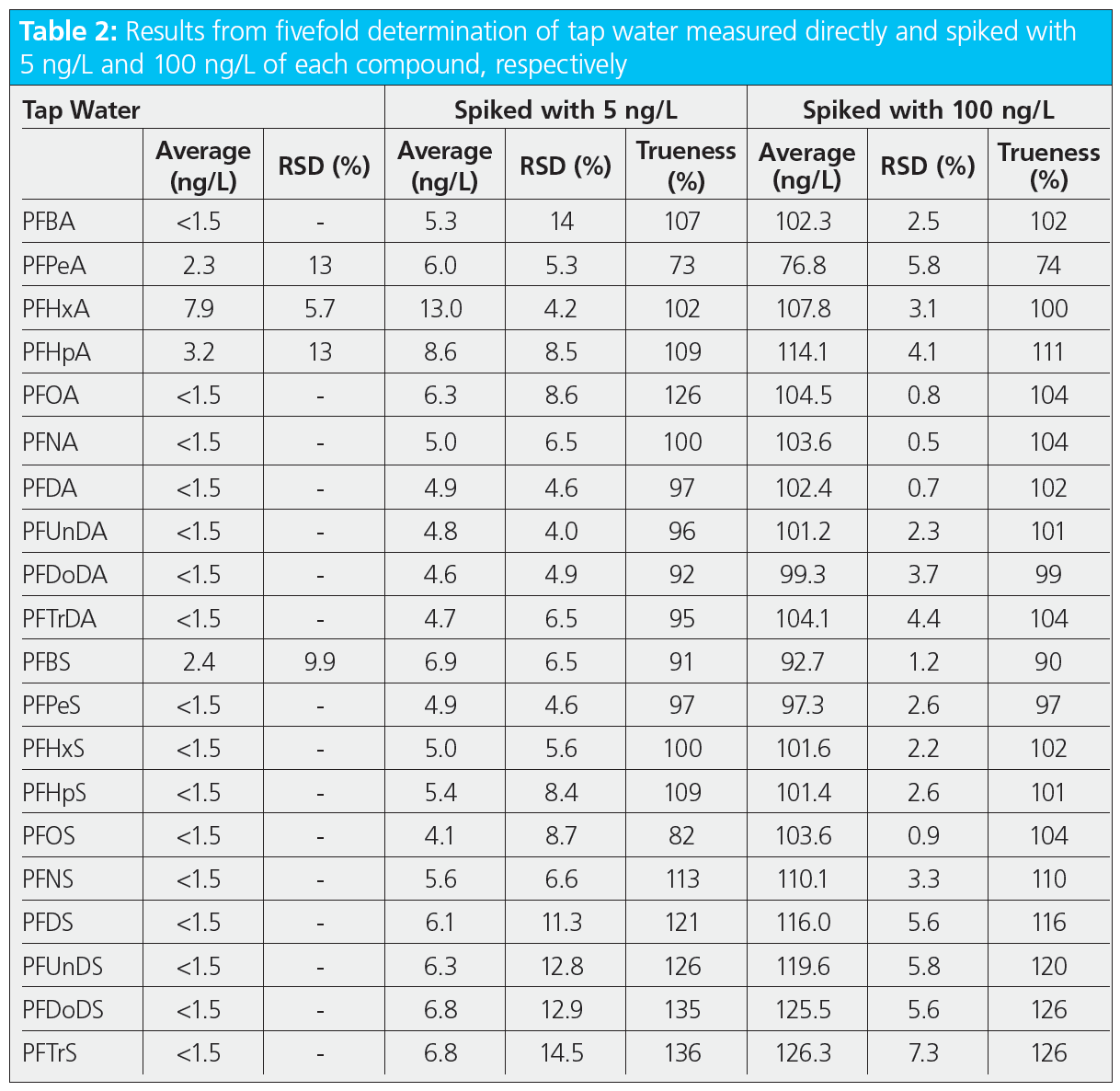
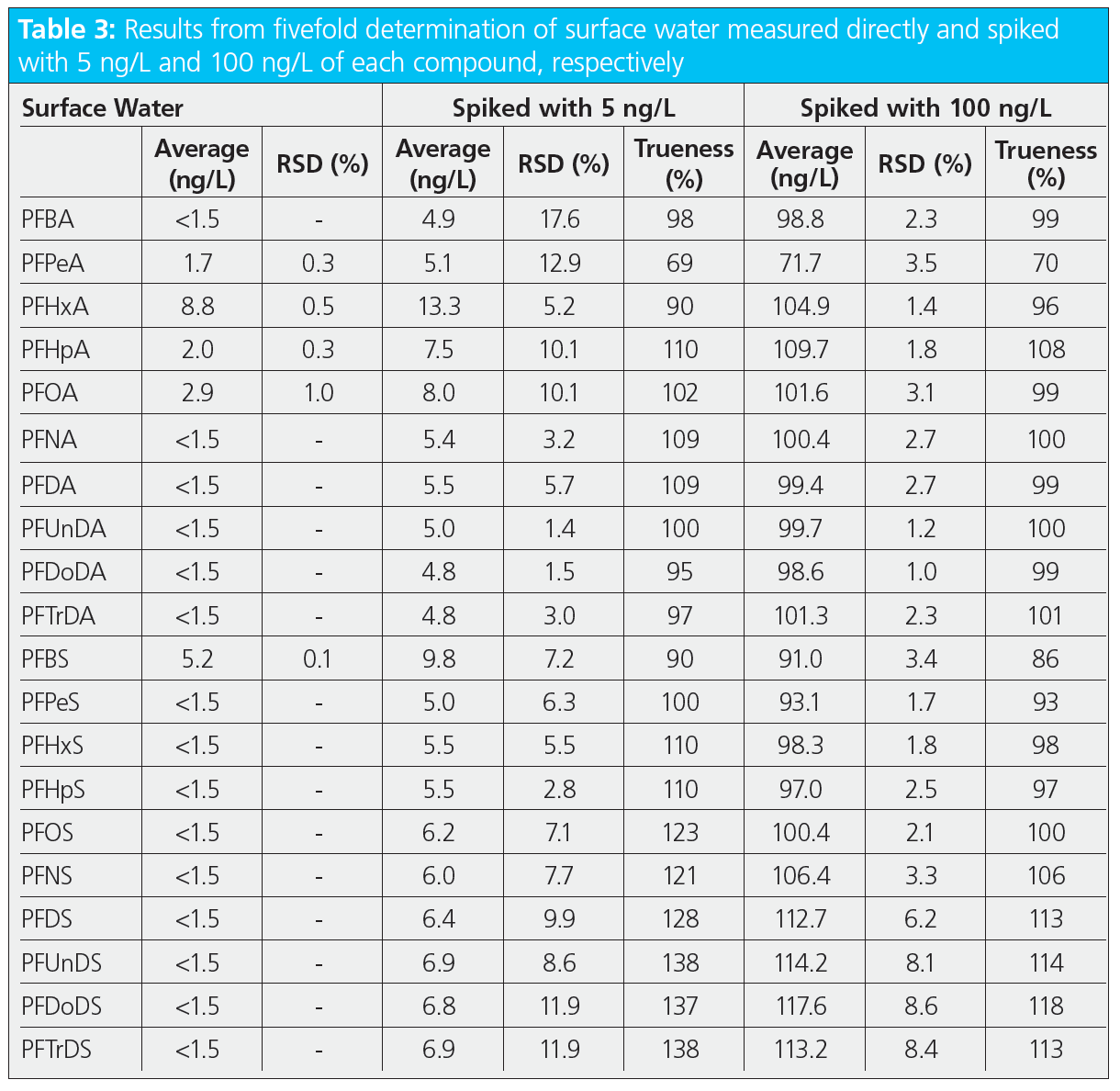
To show the applicability of the method and the trueness of determination, tap water (from our laboratory) and surface water (from the River Ruhr) were measured in replicate and spiked with two concentration levels (5 and 100 ng/L). The results from the fivefold determination of these samples are summarized in Tables 2 and 3. Both samples showed only low levels of some short-chain PFAS (below 10 ng/L). These findings were also confirmed by the concentrations measured in the low‑level spiked samples, for which the trueness was between 90% and 110%, except for PFPeA, which was around 70%. This can be explained by the fact that the properties of this compound, for example, in terms of water solubility, differ from those of the two internal standards used for this region of the chromatogram (mass labelled PFBA and PFHxA). Analysis of the high‑level spiked samples resulted in a trueness between 70% and 130% for all compounds, and relative standard deviations with a median of 2.6% and a maximum of 8.6%, demonstrating the good performance of the presented online‑SPE method.
Conclusions
The online-SPE–LC–MS/MS system combined with the presented method enabled fully automated determination of the PFAS compounds listed in the EU Drinking Water Directive in the low ng/L range, as well as determination following the method described in DIN 38407-42. The main benefits were simple sample handling, very low solvent consumption, and good reproducibility. There was also no need to filter water samples prior to analysis. The SPE system retained particles, keeping them out of the analysis system while enabling the release of particle-adsorbed PFAS for improved overall recovery.
References
- DIRECTIVE (EU) 2020/2184 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 December 2020 on the quality of water intended for human consumption (recast).
- DIN 38407-42 German standard methods for the examination of water, waste water and sludge - Jointly determinable substances (group F) - Part 42: Determination of selected polyfluorinated compounds (PFC) in water - Method using high performance liquid chromatography and mass spectrometric detection (HPLC/MS–MS) after solid-liquid extraction (F42). 2011 Edition, March 2011.
Thomas Brandsch works as an application scientist in the Innovation and Technology Department at Gerstel in the field of automated sample preparation for LC–MS. After receiving his Ph.D. from the Ruhr University Bochum, Germany, in 2003, Thomas worked in positions of increasing responsibility for over 15 years in an environmental contract laboratory, before joining Gerstel in early 2020.
E-mail: Thomas_Brandsch@gerstel.de
Website: www.gerstel.com

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
















