Pesticide and Mycotoxin Analysis: Mastering the Complexity of the Cannabis Matrix
Special Issues
Research scientists in the cannabis field are tasked with validating robust methods that can be seamlessly transitioned into production laboratories. Unlike typical disciplines where controls are easily (and legally) obtained through known manufacturers, analytical chemists working for both consumable vendors as well as cannabis laboratories must do their best to develop methods often without such resources at their disposal. As the industry matures and additional regulations are adopted, the evolution of the pesticide testing subsection continues to be vastly different depending on the jurisdiction one does business in. This creates an interesting challenge for commercial scientists tasked with developing methods that will appeal to a majority of their consumers, while also generating unexpected hurdles to said laboratories once the methods are placed into production. Ace Analytical Laboratory, located in Las Vegas, Nevada, has successfully adopted and validated pesticide testing methods for their cannabis laboratories and has gained valuable insight into how to best work with such a difficult matrix. In conjunction with UCT, LLC, an overview of best practices and method development techniques for pesticide testing in cannabis is discussed below and told from a technical perspective.
Research scientists in the cannabis field are tasked with validating robust methods that can be seamlessly transitioned into production laboratories. Unlike typical disciplines where controls are easily (and legally) obtained through known manufacturers, analytical chemists working for both consumable vendors as well as cannabis laboratories must do their best to develop methods often without such resources at their disposal. As the industry matures and additional regulations are adopted, the evolution of the pesticide testing subsection continues to be vastly different depending on the jurisdiction one does business in. This creates an interesting challenge for commercial scientists tasked with developing methods that will appeal to a majority of their consumers, while also generating unexpected hurdles to said laboratories once the methods are placed into production. This article provides an overview of best practices and method development techniques for pesticide testing in cannabis from a technical perspective.
Unlike other analytical fields, the cannabis laboratory testing industry has yet to reach its maturity. Without cohesive testing requirements among states where the plant is legal, laboratories often struggle to develop coordinated methodologies for the analysis of pesticides and mycotoxins. Although both of these classes of compounds have been widely studied in other matrices, the complexity of the cannabis plant presents additional analytical challenges that do not need to be accounted for in other agricultural products. Up to one-third of the overall mass of the cannabis seed, half of the usable flower, and nearly all of the extracts can be contributed to essential oils such as terpenes, flavonoids, and actual cannabinoid content (1). The biodiversity of this plant is exhibited in the more than 2000 unique strains that have been identified, each with its own pigmentation, cannabinoid profile, and overall suggested medicinal use (2). Although early studies suggested the use of hops as a “matrix-matched” substitution for laboratories beginning method development, experienced technicians quickly realized the two matrices could not be treated as equals.
The struggle to acquire blank, matrix-matched material for reference standards is a problem unique to cannabis laboratories. Clinical and food safety scientists are accustomed to purchasing blank, certified negative biological matrices for all of their testing panels and are even required to pass blind proficiency tests using these matrices. Though studies have been completed on best practices for laboratories analyzing for cannabinoid content in baked goods, cannabis laboratories looking for pesticides and mycotoxins do not have the luxury of simply purchasing (or growing) reference material (3). This hurdle then complicates the establishment of a relevant proficiency testing program. Without having a source for a pesticide-free, legally obtained cannabis flower, most proficiency testing programs are reduced to being essentially a system suitability check with a solvent standard containing the compounds in question (4).
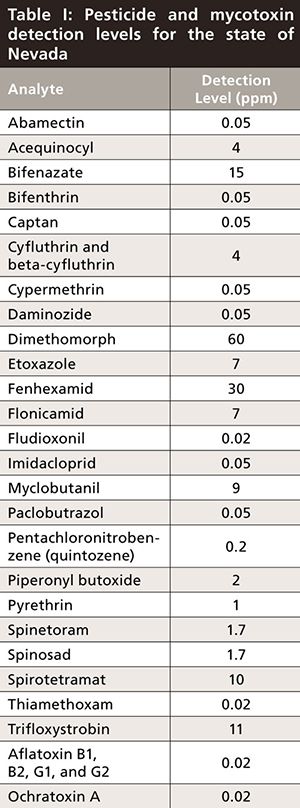
In addition to the complications surrounding the acquisition of a blank matrix, cannabis laboratories also find challenges in sourcing certain pesticide standards. Even after a supplier is identified, the cost of some compounds can prove to be prohibitive to laboratories looking to start up or expand a pesticide panel. Because state law makers have produced banned pesticide lists without consulting the proper scientists, growers, and cannabis experts, many laboratories find themselves attempting to obtain the active ingredients in pesticides that have been banned in the United States for decades. Additionally, some compounds are so unstable in typical extraction conditions that separate methods may have to be developed by the laboratory to comply with state regulations. Even if a reasonable list of pesticides to test for is established within a state, there is no guarantee that the limits of detection (LOD) and the limits of quantitation (LOQ) that need to be demonstrated by each laboratory are meaningful or significant. As shown in Table I, the detection level established in the state of Nevada for abamectin, bifenthrin, captan, cypermethrin, daminozide, fludioxonil, imidacloprid, paclobutrazol, and thiamethoxam are in the parts-per-billion (ppb) range. Such low LOD’s can be extremely difficult, if not impossible, to establish if laboratories do not have extremely sensitive and robust instrumentation. This problem is further complicated by the small batch production common in cannabis, and Nevada does not specify a minimum sample mass to be collected for testing.
The development of an extraction method that can be used in a wide variety of laboratory settings is critical to the emerging field of medicinal marijuana testing. Within environmental and food testing laboratories, the QuEChERS (quick, easy, cheap, effective, rugged, and safe) technique has been widely used for the past 13 years. In 2003, Anastassiades and Lehotay published the first QuEChERS application, which discussed the determination of pesticide residues in produce (5). Since then, QuEChERS has become the analytical gold standard for the testing and analysis of a wide variety of difficult edible matrices, including oil, egg, meat, fish, wine, and beverage samples (5–9). Using disposable consumables, hundreds of pesticides can be analyzed in a single extraction with the QuEChERS approach. In addition to pesticide residues, other chemical classes such as antibiotics, veterinary drugs, mycotoxins, polyaromatic hydrocarbons, bisphenol A, and phthalates are routinely monitored using this technique (6–11). This approach generates much less solvent waste than what is typically associated with complex organic extractions and is a relatively easy analytical method to train technicians on. This advantage allows for laboratories to effortlessly adapt this system of sample preparation with minimal cross-training or cost to the customer and patient.
Traditional solid-phase extraction (SPE) columns and liquid–liquid extraction techniques cannot successfully provide laboratories with the reproducible, cost effective, and fast results needed for cannabis analysis. Unlike several commonly extracted biological matrices, plant-based materials do not easily flow through the porous frits and sorbent of an SPE column. In addition, they do not contain the same endogenous matrix interferences found in biological samples that ultimately need to be removed for accurate quantitation. Liquid-liquid extraction often requires large amounts of undesirable, costly, and toxic solvents to be used, and its overall schematic makes batch processing extremely difficult. The above limiting factors allow for QuEChERS to make a desirable transition to the cannabis laboratory community for pesticide and mycotoxin analysis.
Experimental
Reagents and Standards
High performance liquid chromatography (HPLC)-grade acetonitrile, HPLC-grade methanol, and American Chemical Society (ACS)-grade formic acid were purchased from Spectrum. Neat pesticide and mycotoxin standards were purchased from Sigma-Aldrich, Chem Service, and Ultra Scientific.
Sample Preparation
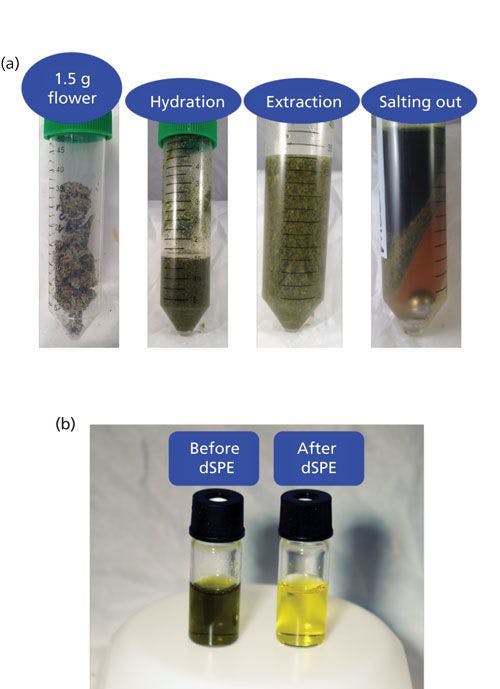
Dried cannabis samples were thoroughly blended into a fine powder using a Robot-Coupe food processor and dry ice. Figures 1a and 1b illustrate a cannabis sample before and after treatment. Homogenized 1-g cannabis samples were weighed into 50-mL polypropylene centrifuge tubes. To each of these samples, 10 mL of ultrapure water was added. Following a brief vortex mixing step, samples were allowed to hydrate for 15 min to improve extraction efficiency. Acetonitrile (10 mL) containing 2% formic acid was then added to each tube. The contents of a Mylar pouch containing an unbuffered QuEChERS blend (4 g of magnesium sulfate and 1 g of sodium chloride) were added, followed by a 5-min shake on a Spex 2010 Geno/Grinder homogenizer at 1500 rpm. All samples were then centrifuged for 5 min at ≥3000 rcf. Dispersive solid-phase extraction (dSPE) was then used for further sample cleanup, including concentration of the pesticides and mycotoxins in a 1-mL aliquot of the resulting organic layer. A blend of magnesium sulfate (150 mg), primary secondary amine (PSA, 50 mg) and ChloroFiltr (UCT Inc.) polymeric-based sorbent (50 mg) was packed into a 4-mL SpinFiltr tube. For comparison purposes, a second blend containing magnesium sulfate (150 mg), PSA (50 mg), and 7.5 mg of graphitized carbon black (GCB) was also used on an additional set of QuEChERS aliquots. PSA assists in the removal of pigmentation and organic acids, whereas magnesium sulfate further removes any remaining water from the extract. ChloroFiltr, which was designed for the selective removal of chlorophyll, was selected because of its effectiveness in removing pigmentation without sacrificing the recovery of planar analytes. GCB is commonly used in agricultural applications for the removal of pigmentation. A SpinFiltr tube (UCT Inc.) was selected for the sorbent blend to be packed into because of its reduction in sorbent carry over and increased sample purification abilities from the inclusion of a 0.2-µm PTFE frit. After transfer to this tube, all samples were vortexed for 30 s followed by centrifugation at ≥3000 rcf for 5 min. The resulting sample was then transferred to an autosampler vial for analysis.
Figure 1: Cannabis sample before (left) and after (right) homogenization with dry ice.

Instrumentation
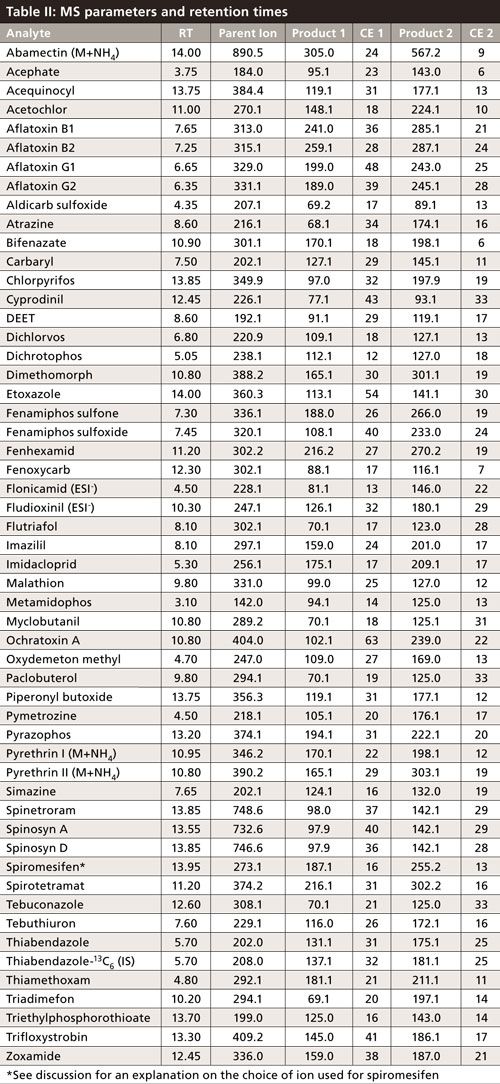
A Thermo Scientific Dionex UltiMate 3000 LC system coupled to a Thermo Scientific TSQ Vantage tandem mass spectrometer was used for all pesticide and mycotoxin analysis. EZ method (scheduled SRM) was used as the acquisition method with Xcalibur (version 2.2) software (Thermo Fisher Scientific) for data processing. A 100 mm x 2.1 mm, 3-µm Selectra Aqueous C18 HPLC column and a 10 mm x 2.1 mm, 3-µm Selectra Aqueous C18 guard column supplied by UCT were used for analyte retention and separation. Although other HPLC packing phases could be used, the polar modification of the aforementioned C18 material proved to be necessary for the retention of some of the extremely polar analytes included in the method. Mass spectrometry (MS) parameters and retention times for all pesticides and mycotoxins are shown in Table II.
The column oven was maintained at 40 °C, the autosampler temperature was 10 °C, and the injection volume of all samples was 5 µL. Mobile-phase A was 5 mM ammonium formate in purified water (Milli-Q, EMD Millipore) with 0.1% formic acid, and mobile-phase B was 5 mM ammonium formate in methanol with 0.1% formic acid. The mobile-phase flow rate was 300 µL/min. The gradient for all sample analysis was as follows: 0 min, 0% B; 2–5 min, 50% B; 5.5–9 min, 60% B; 12–15 min, 100% B; and 15.1–20 min, 0% B. Wash solvent utilized was 1:1 (v/v) methanol–water.
MS/MS was performed with heated electrospray ionization in negative mode (ESI-). The MS/MS system conditions were set as follows: discharge current at 4500 V (ESI-) and 3500 V (ESI-); sheath gas of nitrogen at 55 arbitrary units; auxiliary gas of nitrogen at 40 arbitrary units; ion sweep gas at 0 arbitrary units; vaporizer temperature at 400 °C; ion transfer capillary temperature at 350 °C; collision gas of argon at 1.5 mTorr; Q1 peak width of 0.7 Da full width half maximum (FWHM); and Q3 peak width of 0.7 Da FWHM.
Results and Discussion
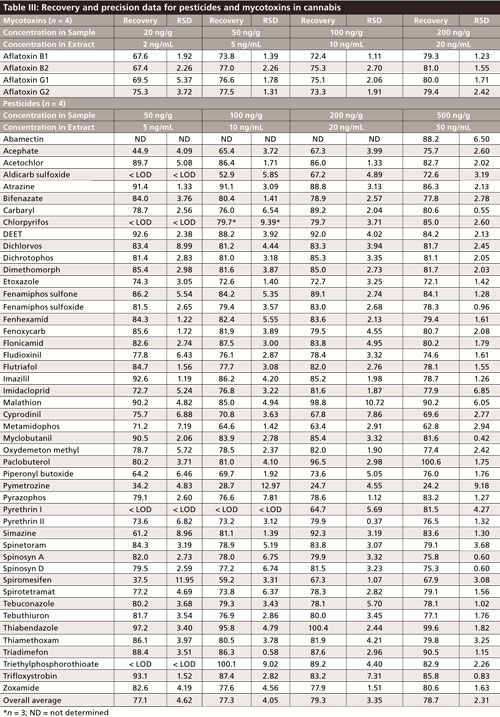
Quantitation was performed against a six-point matrix-matched calibration curve. With the exception of thiabendazole, which had an isotopically labeled internal standard, no internal standards were used for quantitation. All other compounds were quantified using absolute analyte peak areas. Using four replicates, absolute recovery was in the range of 70–100% for most compounds, with reproducibility less than 10% (Table III). The use of GCB was explored as an alternative to the chosen dSPE sorbent blend. Ultimately, better recoveries were obtained using the polymeric sorbent designed for chlorophyll removal (Table IV), with significant differences in recovery highlighted in red. If GCB must be used during the dSPE step, a very small quantity, between 2.5 and 7.5 mg is recommended. Sample cleanliness was similar for both dSPE sorbent blends, as shown in Figure 2. A traditional dSPE vessel, with selected sorbent blend packed into a 2-mL or 15-mL centrifugation tube, was not used to save overall sample extraction time. The use of a larger centrifugation tube with a filter instead allowed for a larger sample to be recovered without a tedious pipetting step.

Figure 2: Comparison of dSPE cleanup between polymeric sorbent for chlorophyll removal and GCB.

CLICK TABLE TO ENLARGE
Acetonitrile containing 2% formic acid and unbuffered QuEChERS salts were selected in the extraction step instead of other salt and acetonitrile combinations to combat the acidic ochratoxin A from being retained on the PSA sorbent during dSPE cleanup. This methodology contributed to the reduced recovery of pymetrozine, a basic analyte, as a result. The acidic pH resulted in insufficient partitioning of pymetrozine into the organic solvent layer.
In spite of this compromise, some ochratoxin A was still retained on the PSA sorbent. Further acidification of the extraction solvent, such as increasing overall formic acid concentration to 5%, may improve the recovery of ochratoxin A. This modification may be explored if this mycotoxin is the most critical compound to the overall success of the extraction. Low recovery and high variability were obtained for ochratoxin A at the lowest fortification level because of the lower signal intensity observed at this concentration. Better results were obtained when evaluating the more highly concentrated samples. This lower signal intensity issue was also observed with acephate, aldicarb sulfoxide, chlorpyrifos, pyrethrin I, spiromesifen, and triethylphosphorothioate at lower concentration levels. Additional liquid chromatography–tandem mass spectrometry (LC–MS/MS) optimization and the inclusion of suitable isotopically labeled internal standards would further improve the overall performance of this method.
Abamectin is prone to sodium (and potassium) adduct formation. The use of an ammonium buffer (acetate or formate) in the organic mobile phase can help to reduce sodium adducting by forming the [M+NH4]+ adduct. Despite this mobile phase selection, the MS signal for abamectin was still quite low and recovery data could only be generated for the highest spiked samples (500 ng/g). Spiromesifen is also an insensitive compound that exhibited a very weak signal for the protonated molecular ion (m/z 371.4). In spite of this, an intense peak at m/z 273.1 was observed, possibly because of in-source fragmentation, and was successfully used for quantitative purposes.
Acequinocyl is an insecticide that is included in the Nevada monitoring list (Table I). Although this compound could be readily detected by LC–MS/MS as a [M+H]+ parent ion when analyzing neat solvent standards, poor results were obtained when conducting spiking experiments and from the matrix-matched calibration curve. Thus, it was omitted from the method. To explain these results, its overall stability was examined. According to Ying and colleagues (12) it undergoes rapid phototransformation in an aquatic environment and rapid hydrolysis under neutral and alkaline conditions. The resulting major transformation product is hydroxyacequinocyl. In the previously mentioned study, it was observed that the highest intensity peak occurred at m/z 343.3, because of the loss of ethenone (CH2=C=O). They also determined that positive atmospheric pressure chemical ionization (APCI+) provided vastly superior signal response over ESI+. For the problematic compounds included in this method, different LC–MS/MS parameters, including interface settings, should be further evaluated to obtain better signal intensity and lower detection limits.
Conclusion
The method outlined above allows for the simultaneous analysis of 47 pesticides and five mycotoxins in cannabis in one simple QuEChERS procedure. By allowing for batch processing, this methodology allows for cannabis laboratories to save time and minimize solvent usage, leading to decreased costs. Analysis of the samples was performed by LC–MS/MS using an aqueous C18 HPLC column, which allowed for improved retention of the more polar pesticides included in the method. The method was evaluated by fortifying cannabis samples with each compound at four levels. The average recovery obtained was generally in the range of 70–100%, and the reproducibility was ≤10%.
With the legalization of cannabis becoming widespread, this simple method is designed for implementation in either start up or established cannabis laboratories that are looking to streamline their sample preparation process, decrease their solvent usage, and provide their clients with accurate and fast results. By using an established extraction technique, such as QuEChERS, laboratories do not need to waste valuable research and development time on perfecting the best sorbent blends and consumables to use. Overall, this method is amendable to various pesticide monitoring lists for states that have already legalized cannabis or for those jurisdictions planning to do so.
References
- “Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products,” United Nations Office of Drugs and Crime (2009).
- W. Ross, Newsweek (2016).
- C.E. Wolf, J.L. Poklis, and A. Poklis, J. Anal. Toxicol. 41(2), 153–157 (2017).
- “Proficiency Testing for Medical Marijuana Independent Laboratories,” Nevada Division of Public and Behavioral Health (2015).
- M. Anastassiades, S.J. Lehotay, D. Stajnbaher, and F.J. Schenck, J. of AOAC Int.86(2), 412–431 (2003).
- S.W.C. Chung and B.T.P. Chan, J. Chromatogr. A1217, 4815–4824 (2010).
- S.C. Cunha, S.J. Lehotay, K. Mastovska, J.O. Fernandes, M. Beatriz, and P.P Oliveira, J. Sep. Sci. 30(4), 620–626 (2007).
- Y. Sapozhnikova, J. Agric. Food Chem. 62, 3684–3689 (2014).
- X. Wang and M.J. Telepchak, LCGC Eur.26, 66–77 (2013).
- J. Wang, and W. Cheung, J. of AOAC Int. 99(2), 539–557 (2016).
- M. Villar-Pulido, B. Gilbert-Lopez, J.F. Garcia-Reyes, N.R. Martos, and A. Molina-Diaz, Talanta85, 1419–1427 (2011).
- X. Ying, H. Cheng, X. Hao, H. Gao, M. Zhang, L. Wu, and H. Wang, Food Anal. Methods8, 578 (2015).
Brian Kinsella, Tina Fanning, Danielle Mackowsky, and Michael J. Telepchak are with UCT, LLC, in Bristol, Pennsylvania. Douglas A. Duncan is with Ace Analytical Laboratory in Las Vegas, Nevada. Direct correspondence to: dmackowsky@unitedchem.com


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)