Analysis of Terpenes in Cannabis Using Headspace Solid-Phase Microextraction and GC–MS
Special Issues
As the legalization of medicinal cannabis continues to sweep across the United States, an urgent need has developed for fast, accurate and efficient analytical testing. In addition to testing for contaminants and potency, there is also interest in the determination of terpene identity and concentration levels present in different strains of cannabis. Terpenes have been shown to have therapeutic uses for treatment of different medical conditions ranging from cancer and inflammation, to anxiety and sleeplessness. It is believed that the combination of terpenes and cannabinoids in cannabis produce a synergistic effect with regards to medical benefits. The traditional testing method for terpenes in plant materials involves a solvent-based extraction followed by GC analysis. In this work, headspace solid phase microextraction (HS-SPME) was used to identify and quantify terpene content in cannabis. The HS-SPME method provided several advantages over solvent extraction in that it provided a cleaner analysis, free of interferences from co-extracted matrix, and was non-destructive to the sample. A cannabis sample of unknown origin was first analyzed qualitatively by HS-SPME and GC-MS. Spectral library matching and retention indices were used to identify 42 different terpenes. Quantitative analysis was then performed for several selected terpenes using spiked samples. Method accuracy was >90%, with reproducibility of
As the legalization of medicinal cannabis continues to sweep across the United States, an urgent need has developed for fast, accurate, and efficient analytical testing. In addition to testing for contaminants and potency, there is also interest in the determination of terpene identity and concentration levels present in different strains of cannabis. Terpenes have been shown to have therapeutic uses for treatment of different medical conditions ranging from cancer and inflammation to anxiety and sleeplessness. It is believed that the combination of terpenes and cannabinoids in cannabis produce a synergistic effect with regards to medical benefits. The traditional testing method for terpenes in plant materials involves a solvent-based extraction followed by gas chromatography (GC) analysis. In this work, headspace solid-phase microextraction (HS-SPME) was used to identify and quantify terpene content in cannabis. The HS-SPME method offered several advantages compared to solvent extraction in that it provided a cleaner analysis, free of interferences from coextracted matrix, and was nondestructive to the sample. A cannabis sample of unknown origin was first analyzed qualitatively by HS-SPME and GC–mass spectrometry (MS). Spectral library matching and retention indices were used to identify 42 terpenes. Quantitative analysis was then performed for several selected terpenes using spiked samples. Method accuracy was >90%, with reproducibility of <5% relative standard deviation (RSD) for analysis of spiked replicates. The HS-SPME results were then compared to an analysis using a conventional solvent extraction method, and the two approaches were found to produce comparable results.
Terpenes are small molecules synthesized by some plants. The name terpene is derived from turpentine, which contains high concentrations of these compounds. Terpene molecules are constructed from the joining of isoprene units in a “head-to-tail” configuration. Classification is then done according to the number of these isoprene units in the structure. For example, monoterpenes contain two units, and sesquiterpenes contain three. The structures of terpenes can be cyclic or open, and can include double bonds, and hydroxyl, carbonyl, or other functional groups. If the terpene contains elements other than carbon and hydrogen, it is referred to as a terpenoid (1). Well known for their characteristic flavors and fragrances, terpenes are contained in the derived essential oils of cannabis. Cannabis contains more than 100 different terpenes and terpenoids, including mono-, sesqui-, di-, and triterpenes, as well as other miscellaneous compounds of terpenoid origin (2). Different cannabis strains have been developed that contain distinct aromas and flavors, which is a result of the differing amounts of specific terpenes present (3). The analysis of cannabis for terpene concentrations can be applied to strain identification, referred to as fingerprinting, and for concentration accuracy when applied to medicinal treatments.
With the growing legalization of medicinal cannabis worldwide, methods for qualifying and quantifying terpene concentrations has risen to the forefront in the analytical industry. As the analytical world tries to keep up with this rapid pace, a need exists for development of faster and more efficient methods that will produce rapid and accurate results at a low cost. Terpenes have high vapor pressures, are extremely volatile, and thus are excellent candidates for static headspace gas chromatography (GC) analysis. Headspace solid-phase microextraction (HS-SPME) offers a low cost and easy alternative to traditional headspace methods. It has been used for both qualitative and quantitative determination of terpenes in a wide variety of matrices, including various foods, beverages, and plant materials (4–7). However, in the analytical testing of cannabis for terpenes, rather than headspace, a common approach has been to use a solvent-based extraction of the plant material followed by GC–flame ionization detection (FID) analysis. In this work, HS-SPME was combined with GC–mass spectrometry (MS) for the qualitative and quantitative analysis of terpenes in cannabis. This approach offers several advantages compared to solvent extraction and GC–FID. It does not require the use of organic solvents, does not coextract matrix (which could potentially interfere with the chromatographic analysis or contaminate the GC system), and provides additional means of peak identification and purity using spectral data. In the first portion of this application, HS-SPME was used to profile the predominant terpenes in an unknown variety of cannabis. A quantitative HS-SPME method was then developed for the determination of several selected terpenes from spiked cannabis samples. The results of this method were then compared to the analysis of the same terpenes using solvent extraction followed by GC–FID.
Experimental
Dried cannabis sample was obtained courtesy of Dr. Hari H. Singh, Program Director at the Chemistry & Physiological Systems Research Branch of the National Institute on Drug Abuse at the National Institute of Health. The extract strain and composition of the sample were not known. The SPME parameters and GC–MS conditions used for quantitative analysis are listed in Table I. All SPME analyses were conducted on a Gerstel MPS 2 autosampler with heated agitator, and an Agilent 6890/5973N GC–MS system operated in scan mode. The qualitative terpene profiling was performed on 0.5 g of dried cannabis weighed into a 10-mL headspace vial, capped, and analyzed directly without any further modification. Terpene identification was done using retention indices and spectral searching against National Institute of Standards and Technology (NIST) and Wiley libraries.
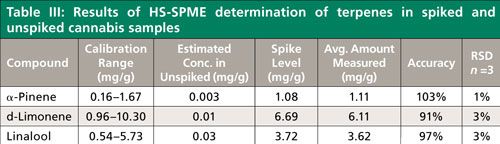
Quantitation was applied to three selected terpenes: α-pinene, d-limonene, and linalool. The strain of the cannabis sample was unknown and HS-SPME analysis of this sample showed it to have a much lower terpene content than what is typically reported for many strains sold for medical and recreational use (8). Thus, to evaluate the HS-SPME method in relevant concentration ranges, the sample had to be spiked with additional amounts of terpenes. The terpene spiking solution was prepared, by weight, from individual neat materials and then diluted into hexane before use. Cannabis samples were prepared for HS-SPME by first grinding the material with a mortar and pestle, and weighing 0.1 g into a 20-mL head space vial. Varying volumes of terpene spiking solution (always less than 10 µL) were added to the dry cannabis to achieve the desired concentrations. After a 10-min equilibration time, 8 mL of deionized water was added to the cannabis, and the samples were placed on the autosampler for HS-SPME analysis. For method evaluation, three spiked samples, two unspiked blanks, and five matrix-matched standards were prepared. Quantitation using extracted ion chromatograms of each terpene was done for spiked samples by external standard against a five-point matrix-matched calibration curve. Matrix spiking was possible because of the low terpene content found to be present in the cannabis used for this study. Consequently, unspiked cannabis was included in the calibration as a zero point.
The solvent extraction procedure used was based on that described by Giese and colleagues (9). Samples were prepared using 0.25 g of ground cannabis weighed into 15-mL polypropylene centrifuge tubes. Spiking solution was added in a similar manner to that described for HS-SPME. After spiking and equilibration, 3 mL of 200-proof ethanol was added, and the samples were vortexed at 2500 rpm for 6 min. They were then centrifuged at 3000 rpm for 10 min, and the resulting supernatant was transferred to a GC autosampler vial for analysis. Three spiked and one unspiked cannabis sample were prepared. Quantitation was done against a five-point calibration curve prepared in ethanol. Samples were analyzed on an Agilent 6890 GC–FID system equipped with an Equity-1 capillary column (60 m x 0.25 mm, 0.25-µm df, MilliporeSigma). The injection and detector temperatures were 270 °C and 300 °C respectively, and the oven was programmed at 60 °C (2-min hold) to 140 °C at 5 °C/min, and to 250 °C at 15 °C/min. Helium carrier gas was used at a linear velocity of 20 cm/s (measured at 40 °C). A 1-µL sample was injected into a 4-mm i.d. tapered FocusLiner (SGE Analytical Science), at a 10:1 split ratio. The terpenes were identified by retention time and quantitated by external standard analysis using peak area.
Results and Discussion
Qualitative Terpene Profiling
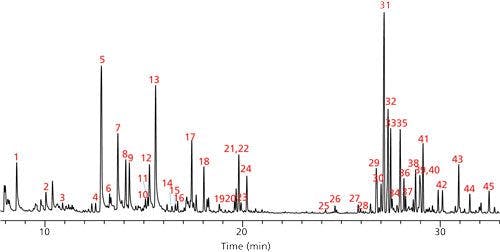
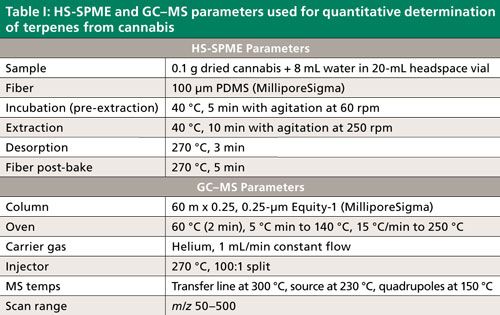
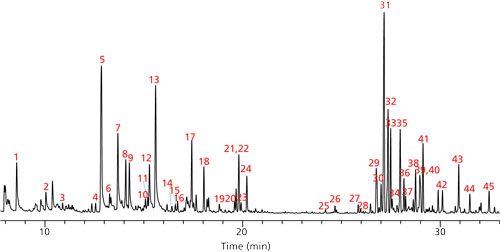
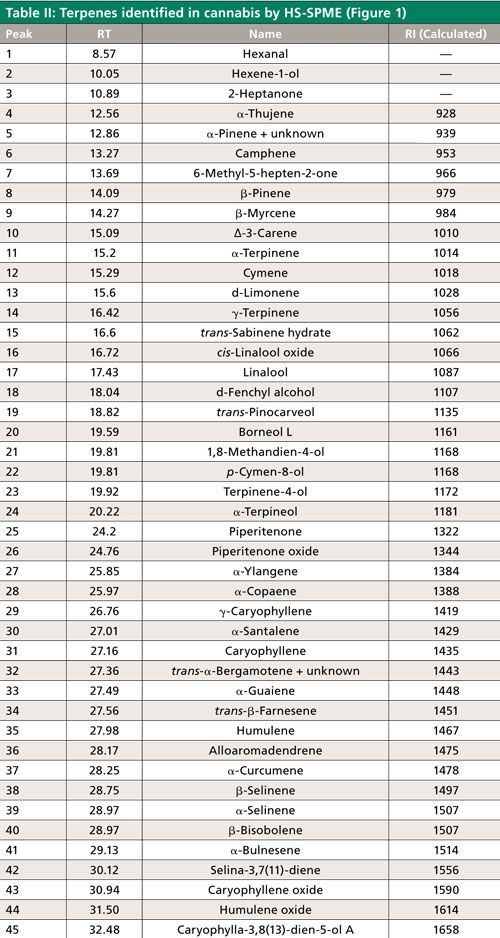
In the first part of this application, we determined the terpene profile of our unknown strain of cannabis by doing HS-SPME directly on the dried sample. To conserve material for future testing, no water was added before analysis. The terpene content of the sample was found to be very low, which required the use of an adsorbent divinylbenzene–carboxen–polydimethylsiloxane (DVB–CAR–PDMS) fiber. This fiber chemistry provided enough sensitivity to produce adequate MS spectra for identification purposes. Peak identifications were then assigned using MS spectral matching against reference spectra in the Wiley and NIST libraries. Since many terpenes have similar MS spectra, additional identification was done based on retention index. Retention indices were calculated for the compounds identified in each sample using an n-alkane standard analyzed on the same GC–MS system under the same GC conditions. This data was compared with published values, and final identifications were assigned, as shown in Figure 1 and Table II (10–12). The terpenes identified were similar to those found previously in the analysis of dried cannabis (10,13). Peaks 1–27 in Figure 1 (with the exception of peak 7) were monoterpenes and monoterpenoids. The later eluting peaks consisted of sequiterpenes and caryophyllene oxide, which is a sequiterpenoid. The most abundant terpene was caryophyllene. The predominance of this compound could be due to the specific strain of cannabis, or the nature of the sample tested, which was dried. Previous studies have shown the level of this compound to increase significantly relative to other terpenes and terpenoids with drying (13). Consequently, the levels of the more volatile monoterpenes and terpenoids would be expected to be less, and this was observed to some degree. Among the monoterpenes and terpenoids the most abundant were α-pinene and limonene.
Figure 1: SPME-HS GC–MS analysis of dried cannabis using qualitative method. See Table II for peak identifications.
Quantitative SPME Method
Method Optimization
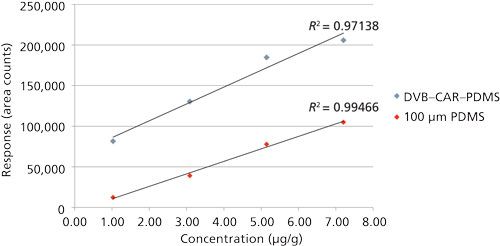
To determine the applicability of SPME for quantitative determination, several key terpenes found in the qualitative analysis were selected for study: α-pinene, d-limonene, and linalool. Initially, the DVB–CAR–PDMS fiber was used, and samples were spiked at levels of 0.3–8.0 µg/g for the three target terpenes, which is 1000x lower than levels reported in typical cannabis samples by testing laboratories (14,15). However, even at this spiking level, calibration curves generated appeared to flatten as concentrations increased, indicating that the fiber was approaching saturation. In addition, fronting was observed for the limonene peak, which confirmed that overload of the chromatographic system was also occurring. Adsorptive fibers such as the DVB–CAR–PDMS fiber have a limited number of binding sites, while absorptive fibers such as polydimethylsiloxane (PDMS) do not, because analytes can diffuse in and out of the polymeric coating. Thus, absorptive fibers can exhibit greater sample capacity, depending on the analyte. A 100-µm PDMS fiber was then chosen for the quantitative method because of its coating thickness and subsequently larger capacity. Compared to the DVB–CAR–PDMS fiber for the same spiking level and matrix, the PDMS fiber showed less indication of overload, as indicated in Figure 2 for α-pinene.
Figure 2: Comparison of DVB–CAR–PDMS and a 100-µm PDMS fiber for extraction of α-pinene by HS-SPME.
Further method optimization was then required to obtain linear response for the three terpenes within a much higher spiking range of 0.1–10 mg/g. Using the 100-µm PDMS fiber, other method variances such as vial and sample size, pre-extraction incubation, and extraction conditions were investigated. SPME is a highly sensitive technique, thus several parameters were optimized to essentially “control” response and thus decrease overload:
- reduce sample weight
- increase headspace volume using a 20-mL vial size
- decrease incubation time
- decrease extraction time
- decrease extraction temperature
- use a split GC injection
While reducing sample weight to decrease response is intuitive, increasing the volume of headspace available above the sample also serves to “dilute” response. Decreasing pre-extraction incubation time reduces the amount of analyte present in the headspace, and reducing extraction time and temperature reduces the amount of analyte absorbed by the fiber. Reducing these parameters can result in extraction occurring under pre-equilibrium conditions. SPME is not an exhaustive extraction technique, and is often referred to as an equilibrium sampling method. However, it should be noted that full equilibrium is not required in all cases to provide quantitative results. Working in a pre-equilibrium state reduces concentrations on the fiber, hence reducing the possibility of fiber overload. When working in this state, precise control of SPME parameters from sample to sample are critical for reproducibility. The use of an autosampler ensured that the same SPME process was repeated exactly on the standards and samples, resulting in the same state of pre-equilibrium, which allowed for reproducible and accurate quantitation. Thus, a 5-min incubation time was chosen for convenience, while reduced extraction times of 2- and 10-min were compared using spiked samples. The longer extraction time provided more reproducible results (data not shown) and thus was chosen for the final optimized method. It was also determined that the addition of water to the dry sample improved extraction reproducibility, especially for linalool. This may be due to an effect on extraction kinetics, as reported by JeleÅ and Gracka for HS-SPME of terpenes from black pepper (16). The final parameter modified to decrease overload was the use of a split injection during desorption of the SPME fiber in the GC inlet. With a splitless injection, overload was evident with all three terpenes, especially at the higher end of the calibration range.
Evaluation of Method Accuracy and Precision
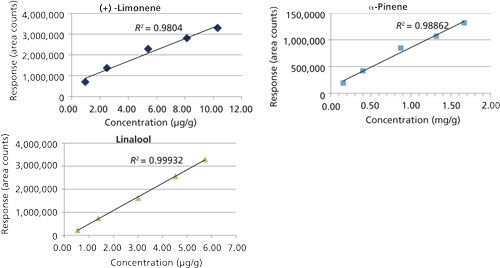
Using the final, optimized method for quantitation (Table I), the cannabis sample used for the qualitative profile study described previously was analyzed. Since the terpene content of our unknown strain of cannabis was well below typical values reported, spikes were prepared to evaluate for method accuracy and precision (14,15). The very low terpene content of our cannabis also allowed its use for preparation of a matrix-matched calibration curve. Others have used pentane extraction to produce cannabis blanks suitable for spiking (9), however this was not necessary in our case. The concentrations used showed good linearity across the calibration ranges for all three terpenes (Figure 3). Using these matrix-matched curves for quantitation, two unspiked and three spiked cannabis samples were analyzed. The concentrations of the unspiked samples were too low to extrapolate from the curves in Figure 3, and were estimated using the lowest matrix-matched calibration standard. This data is presented in Table III, along with the accuracy and precision obtained from triplicate analyses of spiked cannabis samples. Accuracies for the targeted terpenes were >90% with precisions of <5% relative standard deviation (RSD).
Figure 3: HS-SPME analysis of terpene calibration prepared in cannabis.
Comparison of SPME Results to Solvent Extraction
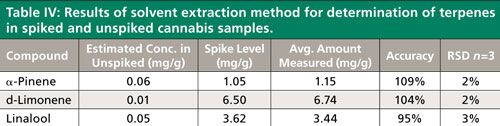
Solvent extraction followed by GC–FID analysis is a fairly common approach to the determination of terpenes in cannabis. Replicate cannabis samples spiked with the three terpenes determined using the HS-SPME method were extracted using ethanol, and analyzed by GC–FID. The results are presented in Table IV. In a comparison of the solvent extraction method to HS-SPME, both produced accurate and reproducible results for the spiked samples. The terpene concentrations of the unspiked cannabis were so low that an accurate determination was difficult by either method using the sample size and parameters described. The main advantage of HS-SPME was found to be minimal “hands-on” sample preparation time, and ease of automation. The total time needed to prepare 10 cannabis samples from weighing to loading onto the automated sampling system was about 25–30 min, including the preparation of calibration standards and spikes. By comparison, the solvent extraction method required about 1 h for standard and sample preparation. The later method required the use of organic solvent (ethanol), consumables, a larger sample size, and preparation equipment such as a multitube vortexer and centrifuge. Since the only reagent added to the cannabis in preparation for HS-SPME was water, the sample could potentially be reused for pesticide residue analysis when using the quick, easy, cheap, effective, rugged, and safe (QuEChERS) approach. In addition, the resulting ethanol extract of cannabis was dark green in color, and after less than 10 injections, the GC inlet liner started to show signs of contamination (Figure 4). Considering the varieties and characteristics of available cannabis strains, this type of contamination resulting from coextracted matrix could potentially be much worse than what was observed with the sample tested here. Finally, the HS-SPME method used MS detection rather than FID. MS detection offers better selectivity and allows for the detection of coeluted interferences, which could affect the accuracy of results.
Figure 4: Ethanol extract of cannabis used for terpene analysis and GC inlet liner after approximately seven injections.

Conclusion
HS-SPME was used in the qualitative and quantitative analysis of terpenes from cannabis. For qualitative analysis, an adsorbent DVB–CAR–PDMS fiber was used to provide a terpene profile of an unknown strain of cannabis. Using both spectral library matching and retention indices, 42 terpenes were identified in the cannabis sample analyzed. The method did not destroy or alter the sample, thus making it potentially available for other testing procedures such as pesticide residue and potency. The quantitative analysis of selected terpenes was then achieved using HS-SPME with a 100-µm PDMS fiber. Accuracies were >90% with precision of <3% RSD for spiked replicates. Although applicability was demonstrated for the quantitation of three selected terpenes, the method could be applied to additional terpenes. HS-SPME provided results comparable to a conventional solvent extraction procedure. However, the former technique offered several advantages compared to the latter, including fewer hands-on steps, no organic solvent, less consumables, and a sample injection into the GC system that is free of interfering background. Finally, GC–MS is recommended for analysis of terpenes rather than GC–FID because of the additional specificity and peak identification it can provide. This approach will prevent errors in quantitation due to coeluting peaks. This study demonstrated the potential of HS-SPME in terpene analysis of cannabis. The demand for cannabis testing will continue to grow as more states in the US legalize it for medical use. Since terpene content is an important part of the profiling of different cannabis strains for medical applications, the need for accurate quantitative and qualitative analysis is extremely important. As presented here, SPME has the potential to provide a means to do both types of testing, and is a green alternative to other methodologies for terpene testing. Additional HS-SPME studies will focus on the analysis of incurred cannabis samples containing higher terpene contents, and the possibility of substituting low-terpene plant materials for terpene-blank cannabis in the preparation of calibration curves.
Acknowledgments
The authors would like to thank Dr. Hari H. Singh, the Program Director at the Chemistry & Physiological Systems Research Brand of the National Institute on Drug Abuse at the National Institute of Health for supplying the cannabis sample. We would also like to thank Bob Shirey and Yong Chen from MilliporeSigma for their helpful discussions on SPME.
References
- R.J. Fessenden and J.S. Fessenden, Organic Chemistry, 2nd Edition (Willard Grant Press, Boston, Massachusetts, 1979), pp. 897–904.
- C.E. Turner, E.G. Elsohly, and E.G. Boeren, J. Nat. Prod. 43, 169–234 (1980).
- B. Rahn, “Terpenes: The Flavors of Cannabis Aromatherapy,” 2014, www.leafly.com/news, accessed 9/18/2015.
- A. Peña-Alvarez, S. Capella, R. Juárez, and C. Labastida, J. Chrom. A1134, 291–297 (2006).
- J.I. Cacho, C.P. Viñas, and M. Hernández-Córdoba, J. Chrom. A1399, 18–24 (2015).
- M.L. Ruiz del Castillom, G.P. Blanch, and M. Herraiz, J. Chrom. A1054, 87–93 (2004).
- A. Sartoratto and F. Augusto, Chromatographia57, 351–356 (2003).
- K. Williams, “Cannabinoids and Terpenoids: The Future of Cannabis Connoisseurship,” 2014, www.leafly.com/news/tags/connoisseur (accessed June 2016).
- M.W. Giese, M.A. Lewis, L. Giese, and K.M. Smith, J. AOAC Int.98(6), 1503–1522 (2015).
- M. Marchini, C. Charvoz, L. Dujourdy, and N. Baldovini, J. Chrom. A1370, 200–215 (2014).
- W. Jennings and T. Shibamoto, Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography (Academic Press, New York, New York, 1980).
- National Institute of Standards and Technology (NIST) Chemistry WebBook, www.webbook.nist.gov/chemistry.
- L.V.S. Hood and G.T. Barry, J. Chrom. A.166, 499–506 (1978).
- Terpene profiling services, www.thewercshop.com/services/terpene-profiling-services, accessed June, 2016.
- www.sclabs.com, view tested samples, accessed 1/13/2017.
- H.H. JeleÅ and A. Gracka, J. Chrom. A1418, 200–209 (2015).
Michael R. Halpenny is an R&D Technician and Katherine K. Stenerson is a Principal R&D Scientist with MilliporeSigma in Bellefonte, Pennsylvania. Direct correspondence to: katherine.stenerson@sial.com

Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.
The Role of SPME Combined with GC–MS for PFAS Analysis
Published: March 25th 2025 | Updated: March 25th 2025Emanuela Gionfriddo and Madison Williams from University at Buffalo, the State University of New York, NY, USA discuss the important role that solid-phase microextraction (SPME) techniques with gas chromatography mass spectrometry (GC–MS) can play in the analysis of per- and polyfluoroalkyl substances (PFAS).