Peak Fronting, Column Life, and Column Conditioning By Ryan D. Morrison and John W. Dolan
LCGC North America
The authors look at three column performance problems encountered frequently in today's laboratory.
This month's installment of "LC Troubleshooting" covers several problems related to column performance. We look at a problem related to peak fronting that appears to be caused by column degradation after several hundred samples are analyzed. This leads to a discussion of just how long a column should last. We wrap up with the other end of column life — how to get the column working right initially.
Peak Fronting
Tailing peaks are almost ubiquitous in liquid chromatography (LC) separations. Over the past 20 years of "LC Troubleshooting," at least a dozen installments of this feature have dealt with tailing peaks. In the days of lower-purity silica, peak tailing often was the result of strong acid–base interactions between basic sample functional groups, such as amines, and acidic silanol groups on the silica surface. One popular way to combat such tailing was to add triethylamine to the mobile phase to saturate the stationary phase's acidic sites. With the widespread use of high-purity silica as the backbone of most of today's reversed-phase columns, tailing problems have been reduced to the extent that amine mobile phase additives are seldom used. Today, silanol tailing remains, but is much less of a problem. Another common cause of peak tailing, column overload, was discussed in last month's "LC Troubleshooting"installment (1).
Peak fronting is much more rare than peak tailing. There is some speculation that complaints are low because peak fronting merely makes a badly tailing peak look more symmetric, but this is a viewpoint for cynics. In our laboratory, we rarely see fronting peaks, and this is likely the problem in most scientists' experience. One classic example of fronting in ion-pair chromatography showed that peak fronting could be eliminated with a change in the column temperature (2). A previous "LC Troubleshooting" column discussed another case in which peak fronting might have been attributable to a void in the column or the mobile phase problems, but the cause of the problem was not definitive (3).
A satisfactory chemical model for peak fronting in reversed-phase LC with most samples is difficult to postulate. However, a problem with the physical structure of the column is more reasonable. An asymmetric void at the head of the column, channeling within the column, or a less dense bed structure along the walls off the column than in the middle, each creates a model that allows one to visualize the fronting process. If a portion of the sample molecules travel through this less dense part of the column, they will travel more quickly, distorting the peak. If the bulk of the peak is retained in the normal fashion, a fronting peak will occur.
We recently observed a case of severe peak fronting that appears to fit the hypothesis of a column void or distortion in the column bed. The LC–tandem mass spectrometry (LC–MS-MS) method uses a 100 mm × 2.1 mm C18 column packed with 5-μm diameter particles designed to work well with 100% aqueous phases. Mobile phase A was 10 mM ammonium carbonate (pH 9.0), and mobile phase B was methanol. An isocratic mobile phase of 5% B was run for 5 min at a flow rate of 0.5 mL/min, followed by an 80% methanol flush. Normally, the method produces chromatograms with symmetric peaks, such as the one shown in Figure 1a. After approximately 500 injections of extracted plasma samples, the chromatograms had deteriorated to the degree shown in Figure 1b. Column reversal was ineffective and normal pressures were observed, suggesting that frit blockage was not the problem source. The column was replaced and the chromatogram was similar to that of Figure 1a. We have seen this failure pattern for several columns, indicating that a column lifetime of approximately 500 injections is typical for this method.
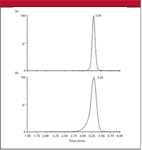
Figure 1: Chromatograms for an LCâMS-MS method. (a) Normal peak shape and (b) severe fronting after approximately 500 injections. See text for details.
Column Life Expectancy
Replacement of the column after 500 injections of the sample above might bring some gasps of horror from some readers. A question we hear frequently relates to how long a column should last. We have methods in our laboratory for which column lifetimes of 2000 injections are common. On the other hand, we've had users tell us of methods in which column failure is observed after 50 or fewer injections — and they are not upset.
Before going into techniques to extend column life, we should consider the cost of the column in the big scheme of things. We work in a contract research organization (CRO), so we make our living from running other people's samples. In the CRO world, LC sample analysis costs generally are $50/sample and up. For our earlier example, the column costs about $500; at 500 samples/column, this translates to $1/sample or 2% of the cost. If we could double the life of the column, we would only reduce the cost to 1%, hardly worth the trouble. However, with a $500 column, many people seem to treat the column as a capital item instead of a consumable. Contrast this with a method that uses solid phase extraction (SPE) for sample cleanup. SPE cartridges or 96-well plates cost at least $2/sample. This is twice the cost of the analytical column, yet we think nothing of throwing the SPE cartridges away after a single use — they are sold as consumable items. Rather than trying to improve column life, a better investment for cost reduction is to figure out ways to reduce labor costs or improve throughput of expensive LC–MS-MS detectors by the use of techniques such as parallel chromatography (4).
Extending Column Life
Although this discussion should convince us that we do not want to spend too much time trying to extend column life, there are some simple techniques that will extend the useful life of a column. The most common mode of failure of columns today is excessive pressure resulting from buildup of particulate matter at the head of the column. Make sure that your samples are free of particulates. Filtration of each sample or centrifugation of samples to eliminate particles will go a long way toward this goal. Another inexpensive, but very effective tool is a 0.5-μm porosity in-line filter mounted just downstream from the autosampler. This will catch the particles that make it past your filtration or centrifugation efforts so that they do not block the column inlet frit. When the system pressure rises to an unacceptable level, simply replace the frit in the filter and you should be back in business. Many workers find that guard columns are beneficial to improving column life. These small columns upstream from the analytical column catch both particulate matter and strongly retained materials that might foul the column packing on the analytical column. If you use guard columns, be sure to discard them before the contaminants break through onto the main column. Also, when flushing the system with strong solvent, be sure to flush the guard column to waste, not onto the analytical column, or you might defeat the purpose of the guard column.
Sample cleanup is another technique to extend column usefulness. You need to remember that there is an economic balance in sample cleanup versus column costs. Does it make sense to spend $10/sample for cleanup to reduce the per-sample cost of the column's contribution from $2/sample to $1/sample? In our opinion, one major goal of sample cleanup should be to improve method ruggedness so that you are assured of collecting meaningful data from your samples. Some workers perform the absolute minimum of cleanup, expect their columns to do the cleanup work, and are satisfied if the column lasts for 100 samples. In other cases, extensive sample cleanup can lower the background noise and allow a 10-fold lower limit of quantification; longer column life is only a peripheral benefit. You have to work out the economics on a case-by-case basis.
Column Conditioning
You might have noticed that some LC methods take a while before they "settle down" and give consistent results. For example, it may take five or six injections before the retention time, peak height, or peak area stabilizes to a satisfactory level of variability. What is going on? Many workers think of a reversed-phase separation as simple partition-like separation between the mobile phase and a homogeneous stationary phase surface. Unfortunately, this is not the case. The closer you look, the less homogeneous the stationary phase appears. In some cases, there are slow and fast equilibria going on at the same time. Sometimes the analyte molecules are retained by more than one mechanism. Loading sample onto the column might allow the slow-equilibrium mechanism to saturate so consistent results are seen. In other cases, it might be proteins, polymers, or other analytically unimportant matrix materials that must be loaded onto the column before the method behaves well. Injection of several mock samples usually will suffice. We have several methods that include five to 10 conditioning injections before injecting the standard curve. This is alright if the runtime is short, but if the method is a conventional LC–UV method, the runtime may be 20–30 min, so extensive conditioning might be unacceptable from a sample-throughput standpoint. Because the conditioning process often is related to the mass of sample (or matrix) loaded on the column, one might be able to shorten the conditioning cycle by making the conditioning injections one after the other without waiting for elution to occur. For example, if the method has a 20-min runtime, just start the method, and then make five injections at 30-s intervals rather than waiting for 20 min for each one. An alternative is to make a single large-mass injection. Try one or more of these techniques and see if it will help your method settle down quickly for normal operation.
Summary
This month, we considered the source of peak fronting problems. Peak fronting is a much less common occurrence than peak tailing. Although there are other possible causes, the most common source of fronting peaks is a disturbance in the column packing bed. The fix is straight-forward: replace the column. Columns should not be expected to last forever. With low-volume assays, a column might last for a year or more, but with high-throughput methods, dirty samples, and harsh mobile phases, column lifetimes can be reduced to 50–100 samples. Remember that the column is a consumable item, so consider carefully how much time and resource to spend in efforts to extend column usefulness. We listed some of the more common techniques to help extend column life: sample filtration, in-line filters, guard columns, and sample cleanup. Some of these, such as in-line filters, are so simple and inexpensive to use, we recommend that they be used on every LC system regardless of other precautionary measures. Finally, we looked at the use of conditioning injections to stabilize the column so that reproducible results can be obtained.
References
(1) John W. Dolan,
LCGC
23
(5), 470–475 (2005).
(2) P.A. Asmus, J.B. Landis, and C.L. Vila, J. Chromatogr. 264, 241 (1983).
(3) C. Hawkins and J.W. Dolan, LCGC 21(12), 1134–1138 (2003).
(4) M.D. Nelson and J.W. Dolan, LCGC 22(4), 338–343 (2004).
Ryan Morrison is an analyst II with BASi Northwest Laboratory, McMinnville, Oregon. His research interests are in method development and validation of LC–MS-MS methods for analysis of drugs in biological fluids.
John W. Dolan
"LC Troubleshooting" Editor John W. Dolan is Vice-President of BASi Northwest Laboratory of McMinnville, Oregon; a Principal Instructor for LC Resources, Walnut Creek, California; and a member of LCGC's editorial advisory board. Direct correspondence about this column to "LC Troubleshooting," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail John.Dolan@Bioanalytical.com.

John W. Dolan
For an ongoing discussion of LC trouble-shooting with John Dolan and other chromatographers, visit the Chromatography Forum discussion group at http://www.chromforum.com.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.














