Optimizing MS-Compatible Mobile Phases for IEX Separation of Monoclonal Antibodies
Special Issues
The impact of ionic strength, buffer capacity, and pH-response on the retention behavior and peak shape of mAb species characterization is evaluated for IEX-MS. The aim of the present study was to understand the impact of ionic strength, buffer capacity, and pH-response on the retention behavior and peak shape of mAb species.
Characterization of monoclonal antibodies (mAbs) and related products requires the identification of chromatographic peaks with mass spectrometry (MS). However, the conventional salt- and pH-gradient elution techniques used in ion-exchange chromatography (IEX) are inherently incompatible with MS. Ammonium acetate- and ammonium carbonate-based mobile phase systems have been recently applied in IEX-MS, but the influence of the eluent composition on peak shape and retention has not been discussed nor studied systematically, until now. The aim of the present study was to understand the impact of ionic strength, buffer capacity, and pH-response on the retention behavior and peak shape of mAb species.
Monoclonal antibodies (mAbs) are a frequently studied group of therapeutic proteins. mAbs are inherently heterogeneous, and their post-translational modifications-such as glycosylation, oxidation, deamidation, or fragmentation, and, particularly, their charge heterogeneity-need to be characterized, because charge variants can be responsible for the efficacy and toxicity of the product (1,2). The two most commonly used methods for charge variant assessment are capillary electrophoresis (CE) and ion-exchange chromatography (IEX) (3–6).
Cation exchange chromatography (CEX) is currently considered to be the "gold standard" method for the separation of main isoform, acidic, and basic variants of mAbs (7–9). In CEX separations, analytes can be eluted by applying either pH-gradient (10–12), salt-gradient (9,13,14,15), or salt-mediated pH-gradient modes (16). Because of the nonvolatile nature of the salts as sodium chloride (NaCl) or potassium chloride (KCl), and buffers as 2-(N-morpholino)ethanesulfonic acid (MES), and phosphate commonly used in the CEX mobile phase, this analytical strategy is not compatible with mass spectrometry (MS). To avoid this issue, Alvarez and colleagues applied a series of trap cartridges for desalting the CEX effluent before entering the MS system (17). Another possibility was to replace common salts and buffer components with MS-compatible buffers. Some research groups recently reported that ammonium acetate and ammonium carbonate (or bicarbonate) buffer systems are particularly promising, and can provide appropriate chromatographic retention and peak shape, together with suitable MS signals (18–21). Those buffer systems have been applied for various mAbs possessing a wide range of isoelectric points (pI). Elution of the mAb products was achieved by increasing the ammonium acetate concentration at constant pH (22,23), or by applying a pH gradient by adding ammonium hydroxide and methanol into eluent B (24). The most promising application was recently presented by Yan and associates, who performed a simultaneous ionic strength and pH gradient to develop a generic method, which can be applied for various mAbs (19). They did this with a gradient wherein the total ionic strength increased from 40 to 150 mM, while the pH changed from 5.6 to 7.4.
The purpose of this article is to understand the impact of pH and ionic strength gradients on the retention of mAbs when working with ammonium acetate and ammonium carbonate buffer systems.
Materials and Methods
FDA- and EMA-approved mAbs were obtained as European Union (EU) pharmaceutical-grade drug products from their respective manufacturers. Ammonium acetate, acetic acid, and ammonium carbonate were purchased from Sigma-Aldrich. High performance liquid chromatography (HPLC)-grade water was obtained from Fisher Scientific.
Ammonium acetate and ammonium carbonate-based buffer systems were systematically tested for mAb separations. The weaker eluent was a mixture of 10-mM ammonium acetate and 10-mM acetic acid (pH~5.2), which maintained a low ionic strength, while the stronger eluent was ammonium acetate and ammonium carbonate mixed in different ratios and concentrations-10 mM, 25 mM, 50 mM, and 100 mM of both acetate and carbonate. Sixteen different combinations of buffer composition were applied, and the retention times of three mAbs (bevacizumab, daratumumab, and rituximab) were measured to study the impact of pH and ionic strength gradients on retention properties. After establishing generic conditions, seven intact mAbs (eculizumab, panitumumab, reslizumab, pembrolizumab, atezolizumab, adalimumab, and rituximab, embracing a pI range from 6.1 to 9.4) were injected and eluted applying an 8 min–long linear gradient. Finally, two gradient separations were further optimized to analyze the mixtures of panitumumab and cetuximab, and nivolumab and ipilimumab. All mAbs were diluted in water at 1 mg/mL, except for the ipilimumab and nivolumab combination, for reasons explained in the results and discussion section.
The separations were performed either on an Agilent 1290 Infinity ultrahigh-performance liquid chromatographic system, or on a Waters Acquity UHPLC H-Class Bio system. A 50 × 2 mm, 5-µm ProPac Elite weak cation-exchanger column (Thermo Fisher Scientific) was employed. Fluorescence detection was performed at 280 nm with a 360 nm excitation/emission wavelength.
Buffer capacity and pH response were calculated for every buffer composition considering the mass and charge balance for the acid and the basic agents through an algorithm written in Python programming language (version 3.7, Anaconda Python Distribution, Numpy package). Retention modeling and method optimization were performed by DryLab 4 chromatographic modeling software (Molnár-Institute).
Results and Discussion
Finding the Optimal Volatile Buffer Composition to Perform mAb Separations
Diverse results were presented in some recent papers regarding the pH, ionic strength, buffer capacity, and conductivity responses of some volatile mobile phase systems-including ammonium acetate, acetic acid, ammonium bicarbonate, and ammonium hydroxide-when developing a gradient program. In addition, some published recipes suffer from the lack of either suitable buffering capacity or appropriate ionic strength. Therefore, some in silico calculations were first performed to predict the buffer capacity and pH response of ammonium acetate, acetic acid, and ammonium carbonate systems. These components were found to be highly promising on the basis of initial screening experiments. Mobile phase A consisted of a mixture of ammonium acetate and acetic acid, while mobile phase B consisted of ammonium acetate and ammonium carbonate. Their individual concentrations varied between 10 mM and 100 mM. Simultaneously, 3 intact mAbs were injected and analyzed in 16 different buffer composition combinations. Retention, peak shape, and selectivity were studied, and the following conclusions could be drawn from the experimental observations and in silico calculations. First, to have sufficient retention to elute the mAbs, and appropriate peak shape, the total ionic strength of the mobile phase B should be higher than 70 mM at the elution of the most retained peaks. Second, the buffer capacity should be maintained above 6 mM. Under these conditions, most mAbs will elute in a pH range between 5.5 and 7.5. In addition, the ionic strength should be kept as low as possible to obtain suitable MS sensitivity.
Further to these results, a salt-mediated pH gradient was also performed to gain an in-peak focusing effect as a result of the salt gradient, and to extend the possibilities of changing selectivity by simultaneously performing a pH gradient. In this combined mode, the elution is based on both salt displacement and on changing the charge state of the mAbs.
All of these needs can be fulfilled by using 10 mM ammonium acetate and 10 mM acetic acid as mobile phase A, and 50 mM ammonium acetate and 50 mM ammonium carbonate as mobile phase B. Figure 1 shows the pH response and buffer capacity of such a buffer system as a function of %B eluent.
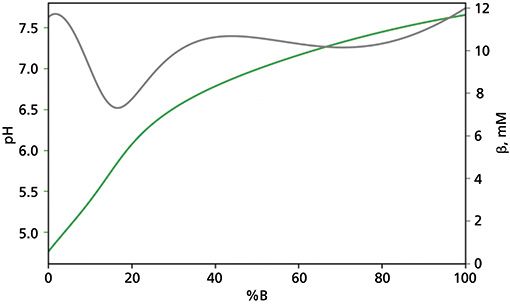
Figure 1: pH (green curve) and buffer capacity (grey curve) responses of the mixture of 10-mM ammonium acetate and 10-mM acetic acid (A eluent), and 50-mM ammonium acetate and 50-mM ammonium carbonate (eluent B) as a function of mobile phase composition (% eluent B).
The impact of ionic strength on retention was studied under various conditions. Figure 2 shows a contour plot of bevacizumab's apparent retention factor (kapp) as a function of ammonium carbonate and ammonium acetate concentrations in mobile phase B. As a side note, the gradient delay volume and system residence time were taken into account when determining the composition at elution. As expected, the ammonium carbonate concentration had a more significant effect on retention than ammonium acetate. This is logical, since the carbonate salt contains twice as much ammonium ion, which is a counter ion, compared to the acetate salt. As an example, a mixture of 10 mM ammonium acetate and 100 mM ammonium carbonate resulted in kapp = 12.6, while the opposite combination of 100 mM ammonium acetate and 10 mM ammonium carbonate provided an almost two times higher retention of kapp = 24.1. In addition, ammonium carbonate is required to ensure the sufficiently high mobile phase pH. On the other hand, ammonium acetate is also required to provide a nearly linear pH response and high enough buffer capacity.
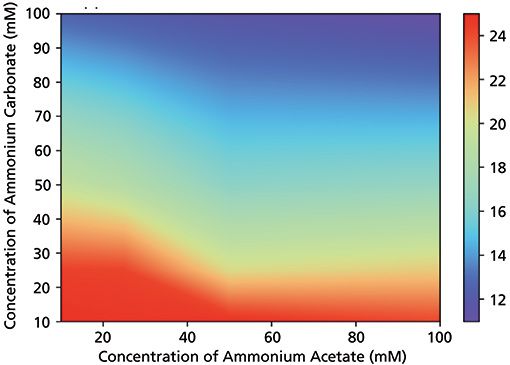
Figure 2: Contour plot of apparent retention factor of bevacizumab as a function of ammonium carbonate and ammonium acetate concentration in mobile phase B. Generic 10-min-long linear gradient (0–100% B) was run at 0.3 mL/min using a 50 × 2 mm cation exchanger column. Mobile phase A consisted of 10-mM ammonium acetate and 10-mM acetic acid.
Figure 3 illustrates the impact of salt concentration (ammonium carbonate) on selectivity. It seems that not only retention but also selectivity is affected by ammonium carbonate concentration. Interestingly, neither the highest (100 mM) nor the lowest (10 mM) salt concentration provided the highest selectivity, but the best selectivity was achieved at 50 mM. By applying 50 mM ammonium carbonate, two basic variants (small peaks after the main peak) and some acidic variants (partially resolved pre-peaks) could be separated. At low concentration of ammonium carbonate, only one sharp peak was observed. It is likely that the steepness of the pH response during the gradient at a fixed gradient composition steepness is the lowest when ammonium carbonate is at 50 mM at elution. On the contrary, the highest pH response steepness was observed with 10 mM ammonium carbonate, and indeed bevacizumab eluted in a single-focused peak. In this latter case, because large solutes approach an "on/off"-like retention, the selectivity was quenched by the steep pH gradient. If the mobile phase composition needs to be further optimized; for example, when the proposed mixture of 50 mM ammonium acetate and 50 mM ammonium carbonate does not provide suitable retention nor selectivity, then the first choice would be to change the ammonium carbonate concentration in mobile phase B.
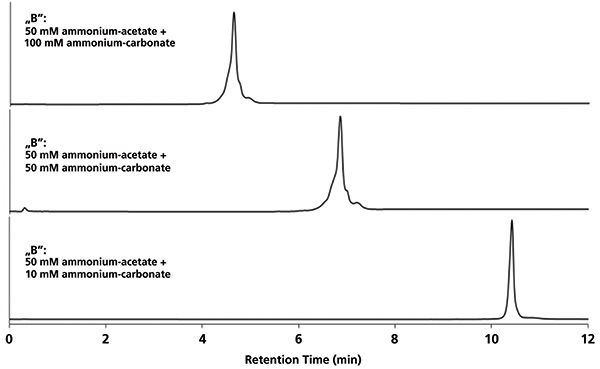
Figure 3: Cation exchanger chromatographic profiles of bevacizumab at different mobile phase B compositions. Generic 10-min-long linear gradient (0–100% B) was run at 0.3 mL/min using a 50 mm × 2 mm cation exchanger column. Mobile phase A consisted of 10-mM ammonium acetate and 10-mM acetic acid.
Generic MS-Compatible IEX Conditions as Platform Method for mAbs
Our proposed mobile phase was also tested for several intact mAbs as candidates for a generic mobile phase of a platform method. The gradient was run from 10 to 70% B, corresponding to a pH range of 5.5 ≤ pH ≤ 7.3. Various mAbs covering a wide range of pI (6.1 ≤ pI ≤ 9.4) could be eluted with appropriate retention, peak shape, and selectivity (Figure 4). mAbs with pI > 7.3 could also be eluted as a result of the high ionic strength of mobile phase B (100 mM in total). The elution order of the mAbs followed their pI, thanks to the nearly linear pH response of the gradient. Therefore, the relative isoelectric points of eluted proteins can be estimated on the basis of their relative retention. Both acidic and basic variants could be separated from the main peaks for most mAbs. The suggested MS-compatible salt-mediated pH-gradient method can therefore be considered as a multiproduct charge sensitive separation method. For most of the compounds, the separation quality was very similar to a pure salt gradient, and slightly better than a pure pH gradient method.
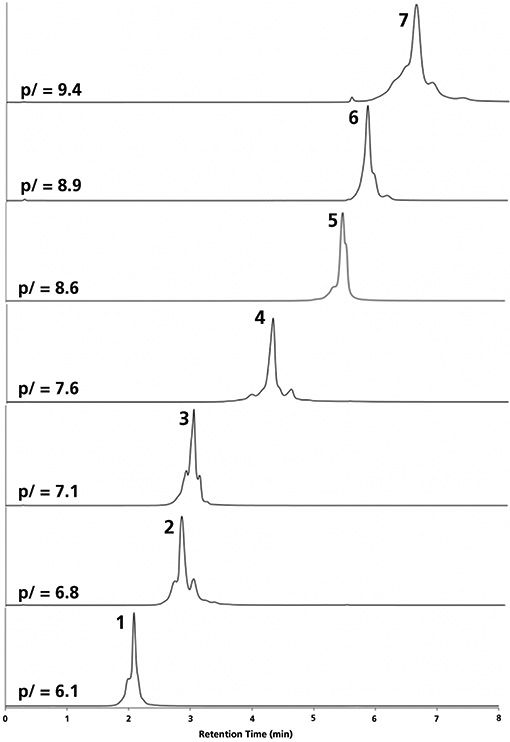
Figure 4: CEX chromatograms of intact mAbs (platform method). Mobile phase A: 10-mM ammonium acetate and 10-mM acetic acid, mobile phase B: 50-mM ammonium acetate and 50-mM ammonium carbonate, gradient: 10–70% B in 8 min, flow rate: 0.3 mL/min, column: 50 mm × 2 mm weak cation exchanger. Peaks: (1) eculizumab, (2) panitumumab, (3) reslizumab, (4) pembrolizumab, (5) atezolizumab, (6) adalimumab, and (7) rituximab.
Optimized MS-Compatible IEX Conditions for mAb Combinations
The combination of different mAbs in one single product could improve the therapeutic efficacy. Two promising combinations are: i) ipilimumab and nivolumab, which have a synergic effect when cytotoxic T-lymphocyte–associated antigen (CTLA) and programmed death 1 (PD1) are targeted simultaneously for the same cancer; and ii) cetuximab and panitumumab, which target the nonoverlapping epidermal growth factor receptor (EGFR) epitopes (25).
Those combinations were mimicked by mixing 1.2 mg/mL ipilimumab and 0.4 mg/mL nivolumab, and 1 mg/mL panitumumab and 1 mg/mL cetuximab. Then, the generic conditions were further optimized to perform either a fast or a high-resolution separation.
Working with salt-mediated pH gradients can significantly extend the design space for method development, when compared to a pure salt or pH gradient. The two most important method variables in salt-mediated pH-gradient CEX are the gradient steepness (proportional to gradient time, tG) and the salt concentrations in the B eluent (16). Here, the salt concentration was fixed at 20 mM in A and 100 mM in B, while varying tG. Experiments were performed at three tG levels (6 min, 10 min, and 18 min, at 0.3-mL/min on a 50 × 2 mm column), and retention models as well as resolution maps were built. The separations were finally optimized based on resolution maps. As an extra note, selectivity can be further increased by changing the salt concentration in mobile phase B, if required.
For the nivolumab and ipilimumab combination, a fast separation was proposed by running a gradient from 32 to 52% B in 6 min, at 0.45 mL/min (Figure 5a). For the other combination of panitumumab and cetuximab, a longer separation was suggested, because both mAbs possess numerous charge variants. In this case, a gradient from 10 to 75% B in 18 min, at 0.3 mL/min, enabled the separation of acidic and basic variants of both mAbs (Figure 5b).
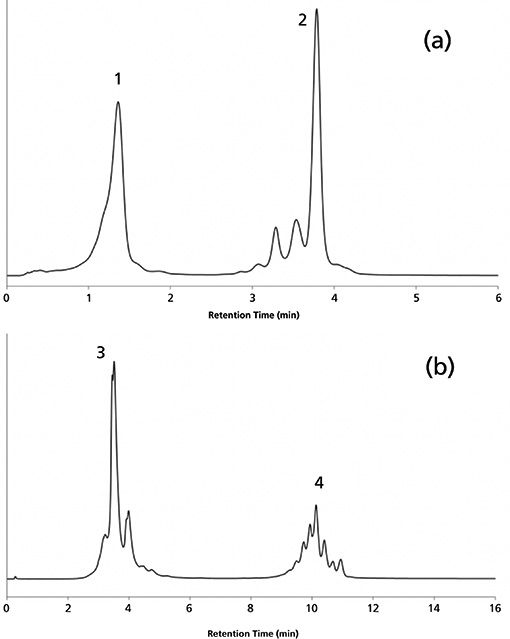
Figure 5: Applications of the suggested MS-compatible mobile phase for mAb combinations: (a) fast separation of nivolumab (peak 1) and ipilimumab (peak 2) and (b) a high-resolution separation of panitumumab (peak 3) and cetuximab (peak 4). See the experimental conditions in “Optimized MS-Compatible IEX Conditions for mAb Combinations” within this article.
Conclusion
This article demonstrated it is possible to establish some generic IEX conditions for charge variants analysis of mAbs, including only volatile components in the mobile phase (MS–compatible). The best performance was achieved using 10 mM ammonium acetate and 10 mM acetic acid as mobile phase A, and 50 mM ammonium acetate and 50 mM ammonium carbonate as mobile phase B. These generic conditions were successfully applied for a wide range of mAbs with pI ranging from 6.1 to 9.4, and for mixtures of mAb products. In an upcoming study, the developed conditions will be tested with MS detection.
Acknowledgements
Davy Guillarme thanks the Swiss National Science Foundation for support through a fellowship to Szabolcs Fekete. Jean-Luc Veuthey from the University of Geneva is also acknowledged for useful comments and discussions. Evelin Farsang and Krisztián Horváth acknowledge the financial support of the Hungarian National Research, Development and Innovation Office (NKFIH FK128350).
References
(1) Y. Du, A. Walsh, R. Ehrick, W. Xu, K. May, and H. Liu, mAbs 4, 578–585 (2012).
(2) H. Liu, G. Gaza-Bulseco, D. Faldu, C. Chumsae, and J. Sun, J. Pharm. Sci. 97, 2426–2447 (2008).
(3) E.D. Lee, W. Mück, J.D. Henion, and T.R. Covey, Biomed. Environ. Mass Spectrom. 18, 844–850 (1989).
(4) J. Cai and J. Henion, J. Chromatogr. A 703, 667–692 (1995).
(5) F. Füssl, K. Cook, K. Scheffler, A. Farrell, S. Mittermayr, and J. Bones, Anal. Chem. 90, 4669–4676 (2018).
(6) P. Dakshinamurthy, P. Mukunda, B. Prasad Kodaganti, B.R. Shenoy, B. Natarajan, A. Mali-Walave, V. Halan, S. Murugesan, and S. Maity, Biologicals 46, 46–56 (2017).
(7) E. Wagner-Rousset, S. Fekete, L. Morel-Chevillet, O. Colas, N. Corvaïa, S. Cianférani, D. Guillarme, and A. Beck, J. Chromatogr. A 1498, 147–154 (2017).
(8) S. Fekete, A.-L. Gassner, S. Rudaz, J. Schappler, and D. Guillarme, TrAC 42, 74–83 (2013).
(9) H. Lau, D. Pace, B. Yan, T. McGrath, S. Smallwood, K. Patel, J. Park, S. Park, and R. Latypov, J. Chromatogr. B 878, 868–876 (2010).
(10) S. Fekete, A. Beck, J. Fekete, and D. Guillarme, J. Pharm. Biomed. Anal. 102, 282–289 (2015).
(11) T. Ahamed, B.K. Nfor, P.D.E.M. Verhaert, G.W.K. van Dedem, L.A.M. van der Wielen, M.H.M. Eppink, E.J.A.X. van de Sandt, and M. Ottens, J. Chromatogr. A 1164, 181–188 (2007).
(12) L. Zhang, T. Patapoff, D. Farnan, and B. Zhang, J. Chromatogr. A 1272, 56–64 (2012).
(13) S. Fekete, A. Beck, J. Fekete, and D. Guillarme, J. Pharm. Biomed. Anal. 102, 33–44 (2015).
(14) V. Joshi, V. Kumar, and A.S. Rathore, J. Chromatogr. A 1406, 175–185 (2015).
(15) A. Farjami, M. Siahi-Shadbad, P. Akbarzadehlaleh, and O. Molavi, Chromatographia 81, 1649–1660 (2018).
(16) E. Farsang, A. Murisier, K. Horváth, A. Beck, R. Kormány, D. Guillarme, and S. Fekete, J. Pharm. Biomed. Anal. 168, 138–147 (2019).
(17) M. Alvarez, G. Tremintin, J. Wang, M. Eng, Y.-H. Kao, J. Jeong, V.T. Ling, and O.V. Borisov, Anal. Biochem. 419, 17–25 (2011).
(18) L. Konermann, J. Am. Soc. Mass Spectrom. 28, 1827–1835 (2017).
(19) Y. Yan, A.P. Liu, S. Wang, T.J. Daly, and N. Li, Anal. Chem. 90, 13013–13020 (2018).
(20) F. Füssl, A. Trappe, K. Cook, K. Scheffler, O. Fitzgerald, and J. Bones, mAbs 11, 116–128 (2019).
(21) Y. Leblanc, C. Ramon, N. Bihoreau, and G. Chevreux, J. Chromatogr. B 1048, 130–139 (2017).
(22) K. Muneeruddin, C.E. Bobst, R. Frenkel, D. Houde, I. Turyan, Z. Sosic, and I.A. Kaltashov, Analyst 142, 336–344 (2017).
(23) K. Muneeruddin, M. Nazzaro, and I.A. Kaltashov, Anal. Chem. 87, 10138–10145 (2015).
(24) M. Talebi, A. Nordborg, A. Gaspar, N.A Lacher, Q. Wang, X.Z He, P. Haddad, and E. Hilder, J. Chromatogr. A 1317, 148–154 (2013).
(25) R. Dienstmann, A. Patnaik, R. Garcia-Carbonero, A. Cervantes, M. Benavent, S. Roselló, B.B.J. Tops, R.S. van der Post, G. Argilés, N.J.Ø. Skartved, U.H. Hansen, R. Hald, M.W. Pedersen, M. Kragh, I.D. Horak, S. Braun, E. Van Cutsem, A.W. Tolcher, and J. Tabernero, J. Cancer Discov. 5, 598–609 (2015).
Evelin Farsang is a PhD student at the University of Pannonia, in Veszprém, Hungary. Amarande Murisier is a PhD student at the University of Geneva, in Switzerland. Krisztian Horvath is an Associate Professor at the University of Pannonia in Veszprém, Hungary. Olivier Colas is a Technician in analytical chemistry at the Centre d'Immunologie Pierre Fabre, in France. Alain Beck is the Senior Director of Biologics CMC and Developability at Pierre Fabre Laboratories, in France. Davy Guillarme is a Senior Lecturer at the University of Geneva, in Switzerland. Szabolcs Fekete is with the Analytical Pharmaceutical Chemistry group at the University of Geneva, in Switzerland. Direct correspondence to: Szabolcs.Fekete@unige.ch

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.






