New Generation Monolithic Silica Columns for Fast, High Resolution Drug Separations without High Pressures
The Application Notebook
Using a novel new-design monolithic silica column (Chromolith? HighResolution RP-18e), fast, high resolution separations were achieved for various drug mixtures without the high pressures characteristic of modern particulate technology.
Monolithic silica technology has evolved as a significant development in fast HPLC analysis over the last decade. As the true alternative to conventional particle-packed column technology, the columns are made of a continuous single piece of high purity porous silica. A sol-gel synthesis process is used, leading to single-particle rod columns, which possess a bimodal pore structure with macro- (flow path) and meso- (active site) pores in the 1 µm and 140 Angstrom range. High performance results from the permeability efficiency of the fixed flow pathways in the highly-controlled porosity of the synthesized rigid silica skeleton. The resulting very low operating backpressures compared to closely-packed spherical particles allow for more flexible flow rates and solvent choice and enable high throughput analysis without loss of separation efficiency and peak capacity. Monolithic silica columns are increasingly being used for quality control of drugs. Easy and cost-effective method transfer offers it great advantages over other trends toward fast HPLC analysis. Flow programming with monolithic columns provides additional reduction of analysis time. Since there is no particle bed to disturb, pressure shocks do not affect its performance or lifetime. The possibility for direct injection of biological fluids without pretreatment has made it more applicable for bioanalysis (1). To date, hundreds of papers have been published describing the use of monolithic columns in various analytical fields which include routine drug analysis and QC, food and environmental analysis, natural products analysis and bioanalysis (2).
In this application note a separation of drug substances is presented. Seven beta-blockers are baseline separated in 4 min at 2 mL/min flow rate with just 76 bar backpressure (Figure 1). Even short columns have sufficient resolution to separate five drugs in < 3 min (Figure 2). Both separations possess high efficiency and high peak symmetry. Both of these applications can easily be adapted to MS detection, by simply adding a post-column flow splitter to a MS detector for further characterization of the API.

Figure 1
Conclusions
Separation of drugs is easily achieved on a newly designed monolithic silica Chromolith® HighResolution columns. This application note illustrates that ultra-high efficiencies could be reached without ultra-high pressures.
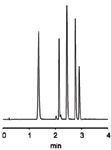
Figure 2
References
(1) E. Machtejevas and K.K. Unger, in Proteomics Sample Preparation, Sample preparation for HPLC – based proteome analysis, Jörg von Hagen, Ed. (Wiley-VCH, Weinheim, Germany, 2008), pp. 245–264.
(2) M. Taha, A. Abed, and S. El Deeb, in Monolithic silicas in separation science, Quality control of drugs, K.K. Unger, N. Tanaka, and E. Machtejevas, Eds. (Wiley-VCH, Weinheim, Germany, 2011), pp. 189–206.
Merck KGaA
Frankfurter Str. 250
D 64293 Darmstadt, Germany

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.




















