Establishing USP Rebaudioside A and Stevioside Reference Standards for the Food Chemicals Codex
The United States Pharmacopeial Convention (USP) publishes the Food Chemicals Codex (FCC), which is a compendium of food ingredient documentary standards (monographs) that provide tests, procedures, and acceptance criteria to indicate the safety and quality of a food ingredient. Physical reference standards can be associated with the procedures of an FCC monograph and the reference standards are needed for conducting the procedures. This article describes characterization and collaborative testing of new USP rebaudioside A and stevioside reference standards and their suitability for intended uses in the FCC rebaudioside A monograph.
The stevia plant contains rebaudioside A and seven or more related steviol glycosides. As a family of related compounds with similar chemical structures and properties, the steviol glycosides form and coexist naturally with different contents in the same plant and have similar but not identical organoleptic features. For example, they are all sweet but to different degrees and with different flavor profiles. Various manufacturers follow different extraction and purification steps, yielding varying amounts of the most prevalent compound, rebaudioside A, or the whole family of steviol glycosides. Figure 1 shows the chemical structure of rebaudioside A, a glycoside of the ent-kaurenoid diterpenoid aglycone family. Analysts require sophisticated tests and specialized knowledge to separate such large and complex molecules from each other or from other plant-derived and similarly large, complex molecules for analysis and characterization. The compositional complexities and analytical challenges also require physical reference standards for comparative test procedures such as identification, assay, and impurity limits to establish the authenticity, purity, and quality of the rebaudioside A. For these intended purposes, USP has released rebaudioside A and stevioside (an impurity) reference standards for stevia products containing no less than 95% rebaudioside A. This article describes the results of characterization and collaborative studies that USP conducted to make these reference standards available.
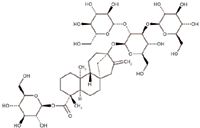
Figure 1: Chemical structure of rebaudioside A (13-[(2-Oβ -D-glucopyranosyl-3-Oβ -D-glucopyranosylβ -D-glucopyranosyl)oxy] kaur-16-en-18-oic acid βD-glucopyranosyl ester).
Experimental
As stated in USP–NF General Chapter USP Reference Standards <11> (1), the establishment and release of USP reference standards are "under the authority of the USPC [United States Pharmacopeial Convention] Board of Trustees upon recommendation of the USP Reference Standards Expert Committee, which approves each lot as being suitable for use in its compendial applications." To support these stipulations, USP scientists generate a set of documents including procurement specifications and a testing protocol and obtain a bulk material, generally from a major manufacturer of the article. The material is tested and characterized in an interlaboratory collaborative study organized according to the testing protocol. The results are evaluated by USP staff, who may conduct additional testing or investigations when necessary. USP staff then compile a report for review and approval by the relevant USP Expert Committee — in this instance, the Food Ingredients Expert Committee. After its approval the material is subdivided as appropriate, quality checked, labeled, and made available for distribution (1).
Liquid Chromatography
Table I summarizes the chromatographic conditions given in the monograph.
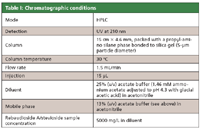
Table I: Chromatographic conditions
To meet specified HPLC system suitability requirements for retention time and tailing factor of the rebaudioside A peak from rebaudioside A standard solution, the monograph allows analysts to adjust the ratio of ammonium acetate to acetic acid in preparing the acetate buffer solution. The adjustment changes the solution pH that affects the retention times of rebaudioside A and other peaks (that is, decreasing the pH of the buffer will decrease the retention time and affect the tailing of the rebaudioside A peak). Other modifications of the chromatographic conditions also may be allowed in a given range.
Characterizing and Establishing Rebaudioside A and Stevioside Materials as Suitable Reference Standards
The collaborative study followed these steps and procedures: We first selected suitable bulk materials of rebaudioside A and stevioside as reference standard candidates that fulfill, at a minimum, all quality requirements of the rebaudioside A monograph. Then we sent samples of these reference standard candidates to three collaborative laboratories that had already received the testing protocols. During the collaborative testing of the two reference standard candidates, all laboratories performed most of the monograph-specified tests whether or not a test required the reference standard. In addition, all laboratories performed additional general tests to characterize and to confirm the chemical structures of the rebaudioside A and stevioside candidate materials, respectively.
Rebaudioside A: The rebaudioside A reference standard is required in two monograph comparative identification (ID) tests: infrared (IR) spectroscopy and high performance liquid chromatography (HPLC) retention time. To comply with the IR test requirement, the IR spectrum of the rebaudioside A sample (product) must exhibit maxima at the same wavelengths as those in the spectrum of the reference standard. To meet the acceptance criteria for the HPLC ID test, the rebaudioside A peak in the sample solution should elute at the same retention time as the rebaudioside A peak obtained from the rebaudioside A (reference standard) standard solution.
The IR spectra of this reference standard candidate by attenuated total reflectance (ATR) analysis are consistent among collaborators, and characteristic IR absorption peaks are observed for functional groups O–H (~3500 cm–1 , broad), C–H for CH2 and CH groups (~2900 cm–1 , multiples), C=C (vinylidine, 1650 cm–1 , weak), C=O (1720 cm–1 , medium), and C–O (1040–1070 cm–1 , strong). The IR data support the chemical identity, and this standard is suitable for its intended monograph comparative IR test.
The rebaudioside A reference standard also is required to verify the HPLC system suitability for the rebaudioside A assay test and for the limit test of organic impurities–related steviol glycosides. Based on the 5000-mg/L rebaudioside A standard solution prepared from rebaudioside A reference standard candidate, the system suitability requirements include specifications that the rebaudioside A peak area and retention time do not vary more than 2.0% relative standard deviation each and that the retention time of the rebaudioside A peak is less than 15.0 min.
Figure 2 shows a typical scaled HPLC chromatogram of rebaudioside A standard solution provided by one of the collaborators. In the chromatogram the retention time of the rebaudioside A peak is about 12 min obtained by the monograph procedure.
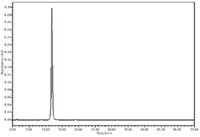
Figure 2: Chromatogram of rebaudioside A reference standard solution at 5000 mg/L.
Quantitatively, the rebaudioside A reference standard is used in the HPLC assay to determine the rebaudioside A content in rebaudioside A product (sample). To comply with the FCC purity requirement, the rebaudioside A content (purity) determined by the assay against the rebaudioside A reference standard must not be less than (NLT) 95.0% on the anhydrous and solvent-free basis. The volatiles, including water and residual solvents (methanol and ethanol), in the material can be determined as directed in the monograph tests.
For qualitative uses such as the ID tests and HPLC system suitability requirements, a labeled purity value for the reference standard may not apply. However, for quantitative applications such as assay and impurity limit tests, users do need to use the purity value of the standard for their calculations. To meet their needs and according to the rule set in USP general chapter <11>, rebaudioside A reference standard as an assay standard is labeled to three significant figures. This reference standard was determined to contain 0.969 mg (96.9%) of rebaudioside A in 1.000 mg of the reference standard material on the anhydrous basis. This value was determined by a standard mass-balance approach by which organic impurities, steviol glycosides, residual solvents, and inorganic impurities are subtracted from 1.000 mg of the reference standard material. Analysts should use this labeled purity value for their assay and related steviol glycosides test calculations as directed in the monograph procedures.
The 96.9% purity is in compliance with the rebaudioside A monograph assay requirement of NLT 95.0%. This lot reference standard also meets the monograph acceptance criteria for impurity limits: ethanol NMT 0.50% (found: 0.1%), methanol NMT 0.02% (not present), related steviol glycosides NMT 5% (found: 3%), and residue on ignition NMT 1% (found: <0.01%).
To determine the hygroscopicity of the rebaudioside A material and the basis for the purity assignment and for reference standard label instructions, vapor sorption and desorption analyses were performed. The analytical data indicated that rebaudioside A is hygroscopic, an observation that was supported by the variation in water content (1–3%) among three collaborators. As a result, for quantitative applications (assay and limit tests), the reference standard label instructs users to determine the water content titrimetrically at the time of use and to use the purity value on the anhydrous basis.
In addition to the IR and HPLC supporting data, the identity and chemical structure (Figure 1) of the rebaudioside A reference standard also has been fully characterized by specific rotation, 1 H and 13 C nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry (MS), and elemental analysis.
The average specific rotation by two determinations was –29.4° (anhydrous), and the monograph acceptance criterion was [α]D25 between –29° and –31° in the initially proposed rebaudioside A monograph. The 1 H and 13 C NMR spectra and their correlations are consistent between two collaborators. The NMR data confirm the chemical structure, and the chemical shift assignments are in agreement with those reported in the literature (2). The MS analysis by direct infusion electrospray ionization (ESI) showed the base peak at 989.64 m/z as the sodium salt adduct of the molecular ion. With the correction of about 2% water content for elemental analysis, the results for carbon and hydrogen contents are consistent with the corresponding theoretical values, which support the chemical composition of this standard.
Stevioside: The stevioside reference standard is used as a qualitative standard for the HPLC system suitability requirements for detector response in the assay and related steviol glycosides tests. It also is used as a quantitative standard for the limit test for stevioside content in the sample.
Based on the monograph assay acceptance criteria for a stevioside reference standard working standard solution at 0.5 mg/L, the detector response by the peak-to-noise ratio (peak height/baseline noise) of the stevioside peak must be NLT 3. This standard met the requirement, and a minimal value of 4 was reported by the collaborators.
For the limit test of related steviol glycosides, we requested collaborators to use the monograph method to analyze the contents of related steviol glycoside impurities in this stevioside reference standard candidate material. The candidate was tested at rebaudioside A sample concentration of 500 mg/L, twice as high as the monograph-specified 250 mg/L for stevioside standard stock solution. Such a high concentration allows more accurate detection of all possible impurities in stevioside material. The average total HPLC impurities are approximately 2% by this analysis.
Figure 3 shows a typical HPLC chromatogram of stevioside reference standard solution with the retention time of the stevioside peak at 6.6 min as suggested in the monograph. Based on the monograph suggested retention times, the two small peaks at 10 min and 12 min were probably because of the presence of rebaudioside F and rebaudioside A, respectively.
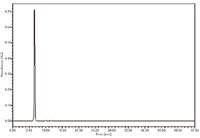
Figure 3: Chromatogram of stevioside reference standard solution at 5000 mg/L.
As a limit standard (1), the purity of the stevioside standard for the current Lot F0I080 was established to be 0.97 mg of stevioside per 1.00 nominal mg of the reference standard material on an as-is basis. The assignment was determined by the mass-balance approach that subtracts all HPLC impurities and volatiles (water and residual solvents).
Unlike the rebaudioside A material, the vapor sorption and desorption analysis data indicate the stevioside material does not appear to be hygroscopic at 50% or less relative humidity (RH). Collaborators also reported consistent water contents. All the data support as-is testing for quantitative uses of stevioside reference standard.
We also requested the collaborators who participated in the rebaudioside A project to perform other similar tests for the stevioside project. In addition to the HPLC data, the identity and chemical structure have been fully characterized by IR, specific rotation, 1 H and 13 C NMR, MS, and elemental analysis. The IR spectra of the stevioside reference standards are very similar to those of the rebaudioside A reference standard, and small differences in peak intensity were observed for the C–O stretches in the 1040–1070 cm–1 region (two or possibly more peaks). Structurally, the stevioside molecule contains one less six-member glucose ring.
The average specific rotation by two determinations was –34.2° (as is), which is different from the value of –29.4° reported for the rebaudioside A. The 1 H and 13 C NMR spectra and their correlations are consistent between two collaborators. The NMR data confirm the chemical structure, and the chemical shift assignments are also in agreement with those reported in the literature (2). The MS analysis by direct infusion ESI (+) showed the base peak at 827.43 m/z as the sodium salt adduct of the molecular ion. With the correction of about 1% water content for elemental analysis, the results for carbon and hydrogen contents are consistent with the corresponding theoretical values, which support the chemical composition of this standard.
Conclusion
The USP reference standards for rebaudioside A and stevioside have been fully characterized by IR, NMR, MS, and elemental analysis, and have been established by collaborative studies for suitability as new USP reference standards. These two new reference standards support the FCC rebaudioside A monograph test procedures for identity (authenticity) and purity (rebaudioside A content and other steviol glycoside impurities). Both the documentary standard in FCC (rebaudioside A monograph) and the newly available rebaudioside A and stevioside USP reference standards support manufacturers, purchasers, and regulators in ensuring the identity, purity, and quality of stevia-containing food and beverage ingredients. These standards help ensure the safety and quality of those ingredients and products throughout the supply chain, which ends with use by a consumer.
Acknowledgments
The authors thank William Koch, Susan S. de Mars, and Roger L. Williams for constructive reviews and Stefan Schuber for editorial assistance. All are colleagues at USP.
Yi Dang, Jeffrey Moore, Markus Lipp, Barbara Jones, and James C. Griffiths are with the U.S. Pharmacopeial Convention, Rockville, Maryland. Direct correspondence to: yd@usp.org for reference standards and jm@usp.org for monograph information.
Gloria Huang is currently at the U.S. Food and Drug Administration. The opinions expressed here are those of the authors and do not represent the official position of the U.S. Food and Drug Administration or the U.S. government.
References
(1) USP, USP Reference Standards <11>, USP 32–NF 27 (USP, Rockville, Maryland, 2009), pp. 35–58.
(2) A.S. Dacome, C.C. da Silva, C.E.M. da Costa, J.D. Fontana, and S.C. da Costa, Process Biochem. 40, 3587–3594 (2005).

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.

















