Native Anion Exchange Chromatography Coupled to Mass Spectrometry for the Charge Variant Analysis of IgG4-Based Monoclonal Antibodies
Ion exchange chromatography is a standard method for characterising monoclonal antibodies (mAbs) and ensuring their efficacy. This article discusses the advantages of a native anion exchange method coupled to mass spectrometry for charge heterogeneity analysis of immunoglobulin G4 (IgG4)-based mAbs, currently being studied more intensively. It also explains why cation exchange chromatography is better suited for the more common IgG1-based mAbs, but less suitable for the IgG4-type mAbs.
Monoclonal antibodies (mAbs) are immunologically active proteins, usually based on immunoglobulin G (IgG) molecules. mAbs target certain antigens that are found on, for example, cancer cells (1). This stimulates the immune system to attack those targets. mAbs are becoming increasingly important for the treatment of different haematologic, immunologic, oncologic and infectious diseases.
A wide variety of therapeutic antibodies are available on the market, and several hundreds more are in research and development (2). To ensure the efficacy of mAbs, quality attributes have to be strictly controlled. These include charge heterogeneity, which usually arises from post-translational modifications. Ion exchange chromatography (IEX) and capillary electrophoresis (CE) are commonly used to characterize the overall charge heterogeneity.
Cation exchange chromatography (CEX) is an excellent method of characterizing the charge heterogeneity for most commercially available mAbs. They are often IgG1-based and possess a high isoelectric point (pI) of usually ≥ 8 (3). Therefore, CEX is the traditional approach; also, coupling of CEX to mass spectrometry (MS) has been successfully described (4). In contrast, anion exchange chromatography (AEX) has only been used for relatively acidic proteins such as human serum albumin (5) or ovalbumin (6). However, for IgG4-based mAbs, which are becoming more important as therapeutic candidates, AEX may be an alternative approach. They possess a pI < 8 and therefore CEX is less suitable (3).
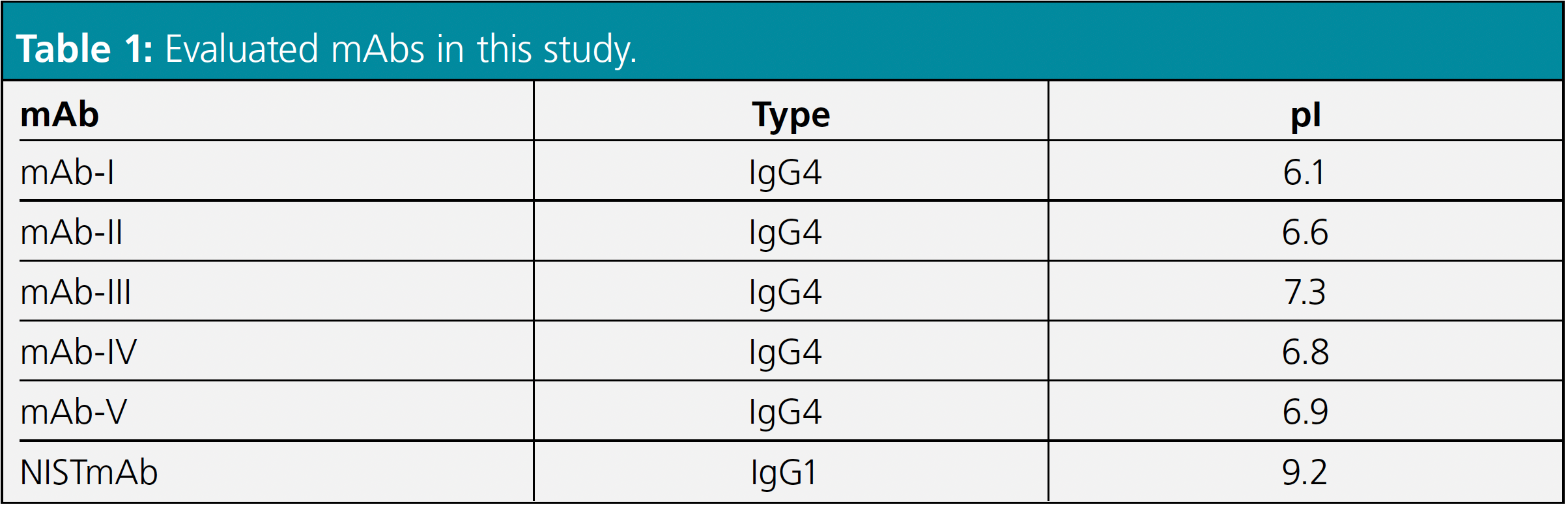
The successful application of an AEX method for charge heterogeneity analysis of IgG4-based mAbs coupled to MS was achieved by Liu and colleagues from Regeneron (7). Their approach is discussed here: Five different IgG4-based mAbs with different pIs (between 6.1–7.3) as well as the NISTmAb (pI=9.2) were analysed (Table 1) using a strong anion exchange column (SAX) with non-porous particles.
Experimental
Native AEX–MS Method Conditions:
The combination of AEX and MS requires a special setup in which a stainless-steel T-piece after the column divides the flow. Most of the flow is directed to the UV detector, while the remaining sub-microlitre per minute flow is directed to the nanoelectrospray ionization mass spectrometer (NSI-MS). NSI is used because it can tolerate high salt concentrations of up to 600 mM ammonium acetate. To further improve the spray stability, isopropanol is used as a dopant, modified desolvation gas.
In addition to the MS detection, the general method conditions were kept constant as follows: Flow rate 0.4 mL/min and 10 μg mAb sample injection unless stated otherwise. A non-porous 100 × 4.6 mm, 5 μm strong AEX column was used (BioPro IEX QF, YMC). A salt gradient from 10 mM ammonium acetate (pH 6.7 unadjusted) (A) to 300 mM ammonium acetate (B) (pH 6.8 unadjusted) is used to analyse IgG4-based mAb samples (in-house mAbs from Regeneron).
The gradient started at 0 %B and was held for 2 min; the ratio of B was then increased to 100% in 16 min and again held for 4 min. The temperature was set to 45 °C, as a result of a previous temperature screening using an IgG4-based mAb at 25 °C, 35 °C and 45 °C. Significant improvement of the variant separation as well as sharper peaks was obtained at the highest temperature (not shown).
Native CEX–MS Method Conditions:
mAb-IV with a pI of 6.8 was used to compare AEX–MS and CEX–MS. For the CEX analysis the same chromatographic conditions were applied as for AEX except for the column and eluents. A non-porous 100 × 4.6 mm, 5 μm strong CEX column (BioPro IEX SF, YMC) was used. The eluents used for CEX were the following: (A) 20 mM ammonium acetate, pH adjusted to 5.6 with 20 mM acetic acid and (B) 150 mM ammonium acetate (pH 6.8).
Analysis of mAbs Using Native AEX–MS Conditions
These conditions were applied to various IgG4-based mAbs (Figure 1) as well as to the IgG1-based NISTmAb. Here, only 5 μg mAb sample is injected. It is shown that the AEX–MS method is suitable for IgG4-based mAbs with moderate pIs, but not for IgG1- based mAbs with higher pIs. The separation improves as the pI gets lower. mAb-III has a pI of 7.3, which is higher than the mobile phase pH, but sufficient separation still occurs. This suggests that it is the surface charge rather than the intrinsic charge that causes the AEX-based separation. In general, the acidic variants separated correlate with those commonly observed by CEX such as deamidation, glycation and sialic acid (Neu5Ac)-containing species. Deamidated variants were found in several peaks. The additional abundant peak A1 of mAb-I contains deamidation that correlates with a known deamidation site in its complementary determining regions (CDR).
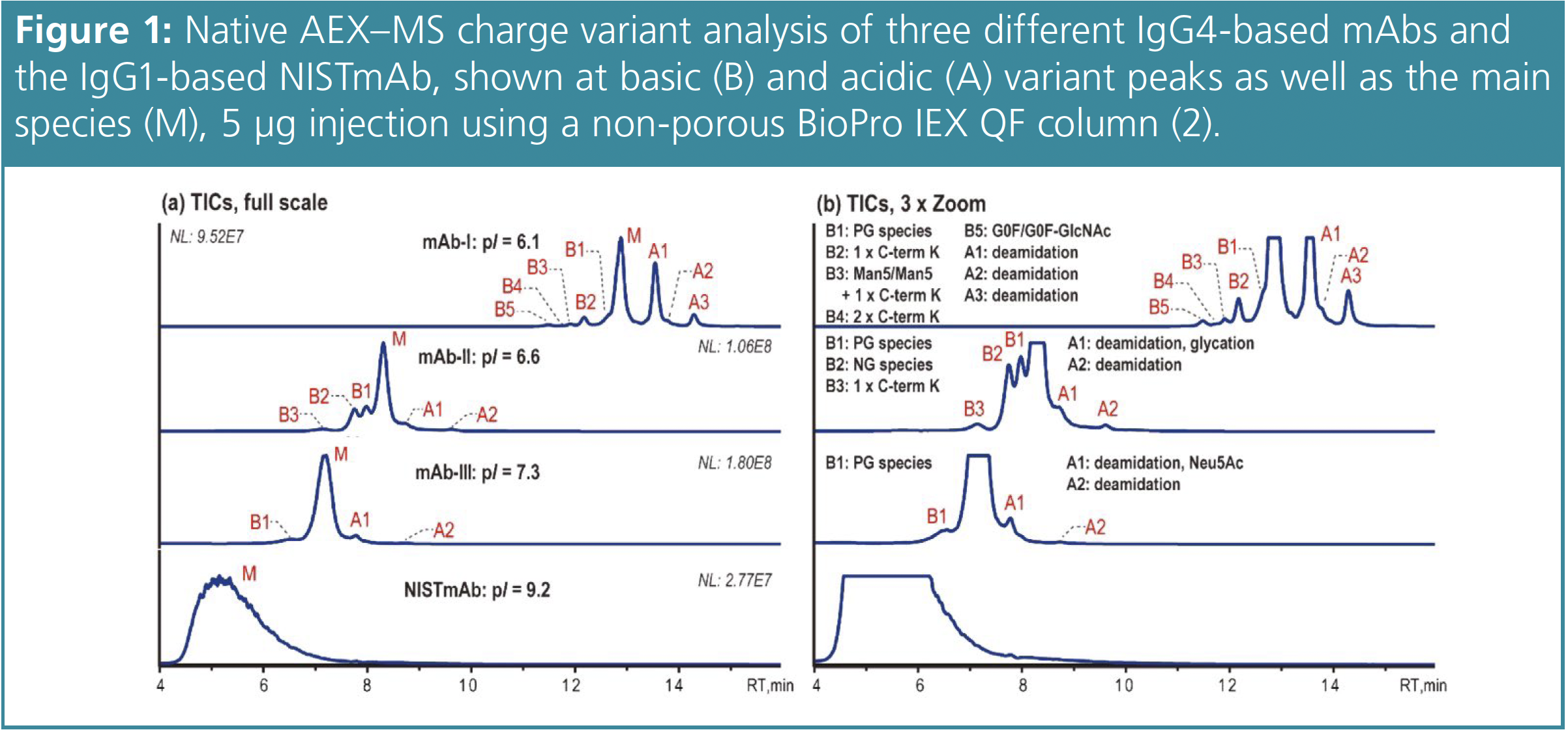
However, the other mAbs tested have no deamidation sites in their CDRs, so these two acidic peaks are probably due to site-specific deamidation in the fragment crystallizable (Fc) region. The basic variants observed can be identified as unprocessed C-terminal lysine (C-term K) and mAb species with different numbers of Fc N-glycans. In addition, the glycoforms Man5/Man5 with unprocessed C-term K and G0F/G0F-GlcNAc are observed for mAb-I. mAb-II demonstrates that this AEX–MS method is very sensitive to the macroheterogeneity of Fc N-glycosylation. The main fully glycosylated (FG) species is separated from the partially glycosylated (PG) peak B1 and the non-glycosylated (NG) species B2, which are eluted earlier.
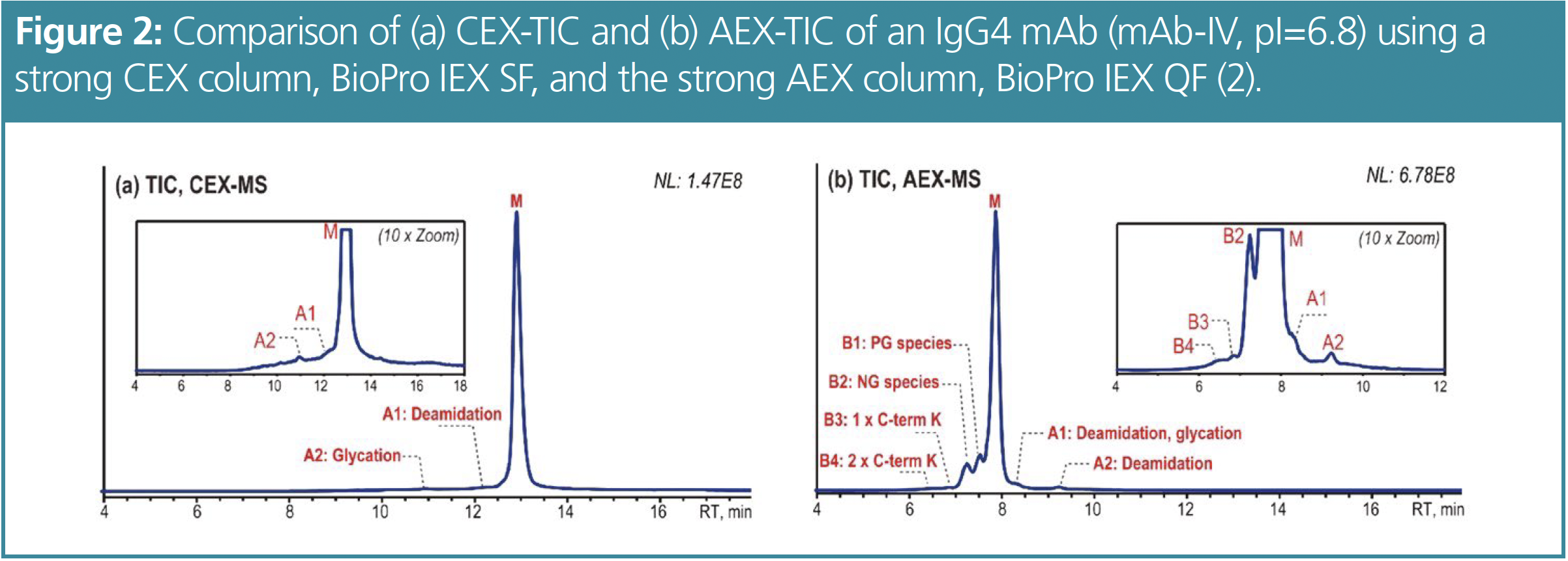
Comparing AEX–MS and CEX–MS
From the CEX–MS method, two acidic peaks can be observed and identified as deamidated (A1) and glycated (A2) variants (see Figure 2). No basic peaks were detected. In comparison, the AEX–MS analysis shows four basic peaks including partially (B1) and non-glycosylated mAb (B2) species and 1 (B3) or 2 unprocessed C-term K (B4) in addition to two acidic peaks. However, A1 consists of a deamidated and glycated variant and A2 contains another deamidated species. The use of AEX–MS probably provides the possibility to separate site-specific deamidation variants. The overall separation using AEX–MS is better as the method provides sharper peaks and additional information about the basic variants.
Further Improvement of the Basic Variant Separation
Since mAbs with a lower pI are better separated, a test was performed to see whether the separation can be improved by lowering the pI through PNGase F (peptide:N-glycosidase F)-mediated deglycosylation. This reaction removes N-glycans and simultaneously converts the glycan-bearing asparagine (Asn) residue to aspartic acid (Asp). Since all IgG4 mAbs contain an Asn residue in the Fc region of each of the two heavy chains, up to two Asn to Asp conversions can be expected. Therefore, the mAbs were treated with PNGase F at 1 IUB milliunit per 10 μg sample in 100 mM Tris-HCl buffer (pH 7.5) at 45 °C for 1 h.
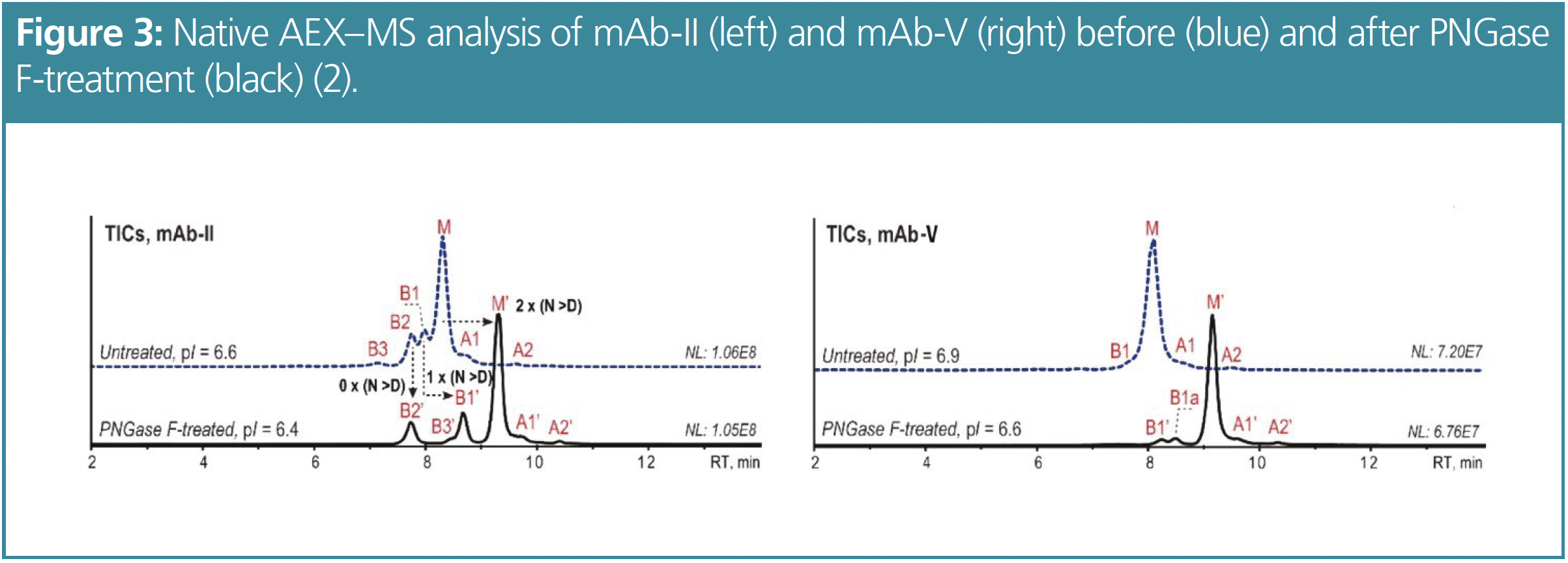
Figure 3 shows the differences between the untreated and the PNGase F-treated mAbs mAb-II (pI=6.6) and mAb-V (pI=6.9). The PNGase F-treatment decreased the pI to 6.4 for mAb-II and to 6.6 for mAb-V. Due to the reduced pI, the overall retention as well as the variant separation and peak sharpness are improved for both mAbs. After the PNGase F-treatment of mAb-II, the retention time of the FG main species increases by about 1 min due to the elevated acidity induced by the now two Asp residues. The retention time of the PG species B1 shifts by only about 0.5 min, while the retention time of the NG species B2 remains unchanged. In the case of mAb-V, an additional minor variant (B1a) can be detected and identified as a partially glycosylated species.
Monitoring Critical Fc Quality Attributes
This AEX–MS method can also be used for subunit analysis of mAbs after digestion with IdeS (immunoglobulin G-degrading enzyme of Streptococcus pyogenes) protease. The already deglycosylated mAbs were treated with IdeS 1 IUB milliunit per 1 μg mAb sample in 100 mM Tris-HCl buffer (pH 7.5) at 37 °C for 90 min to release the Fc and F(ab’)2 subunits. Since the pI of F(ab’)2 fragments is relatively high, these fragments are poorly retained and are not taken into account further.
In contrast to previous results, a lower temperature of 25 °C showed improved peak shape and charge variant separation for the Fc fragment analysis compared to analysis at higher temperatures, as shown in Figure 4 for mAb-II. Four basic and two acidic variants can be identified. Both the main peak as well as the B1 peak show tailing shoulder peaks, with identical mass to the corresponding peak, which could be conformational isomers.
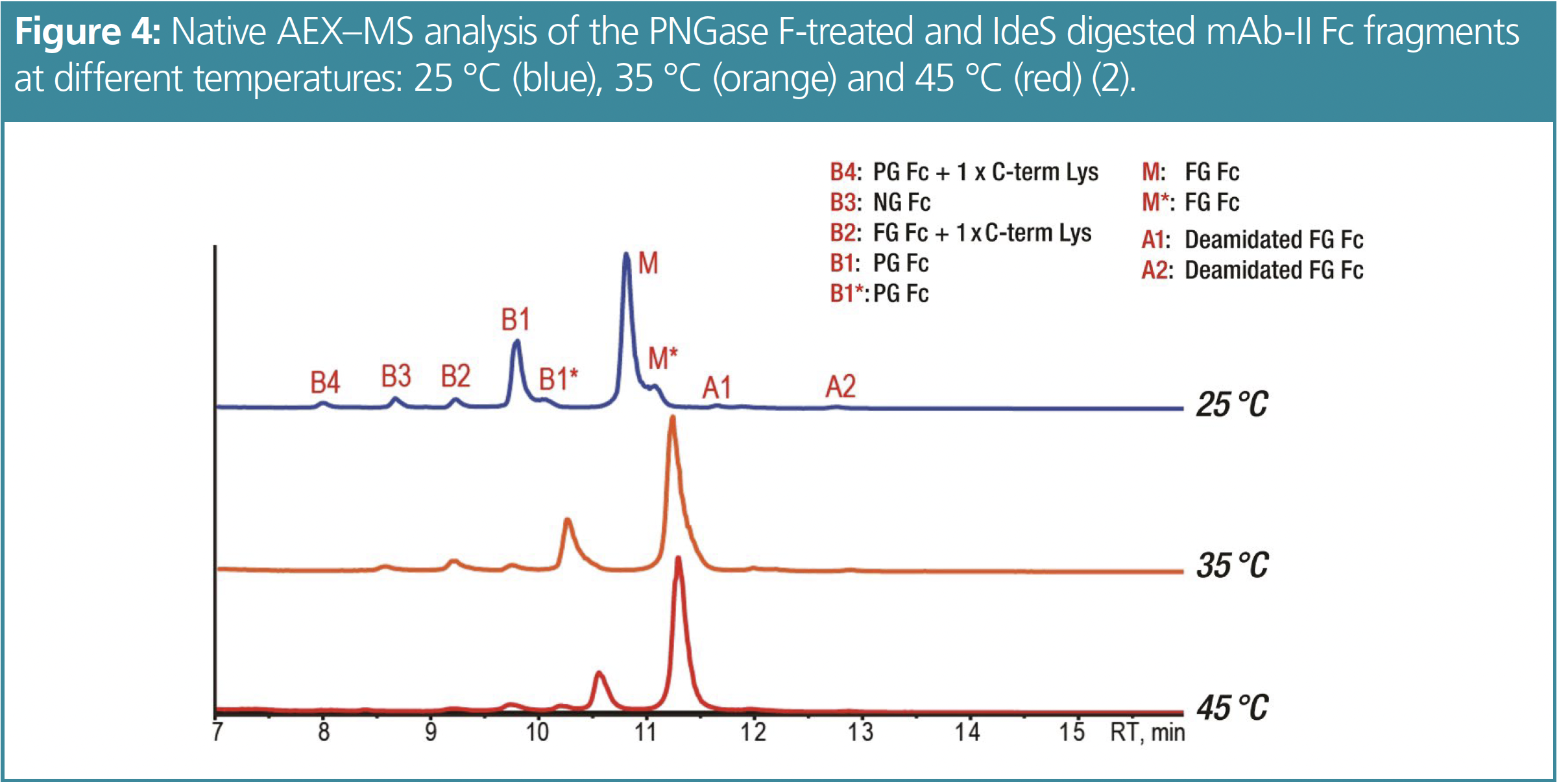
In addition to the NG B3 and the PG B1 peak, B2 is identified as a fully glycosylated species with one unprocessed C-term K while B4 is identified as a partially glycosylated species with one unprocessed C-term K. These findings were confirmed by peptide mapping.
Identification of Site-Specific Deamidations After Thermal Stress
mAb-V was thermally stressed to achieve higher levels of deamidation and to facilitate fractionation after IdeS digestion and deglycosylation. Therefore, mAb-V was incubated at 25 °C for 6 months. The basic Fc variants of mAb-V are also assigned to the unprocessed C-term K and Fc N-glycosylation macroheterogeneity (see Figure 5).
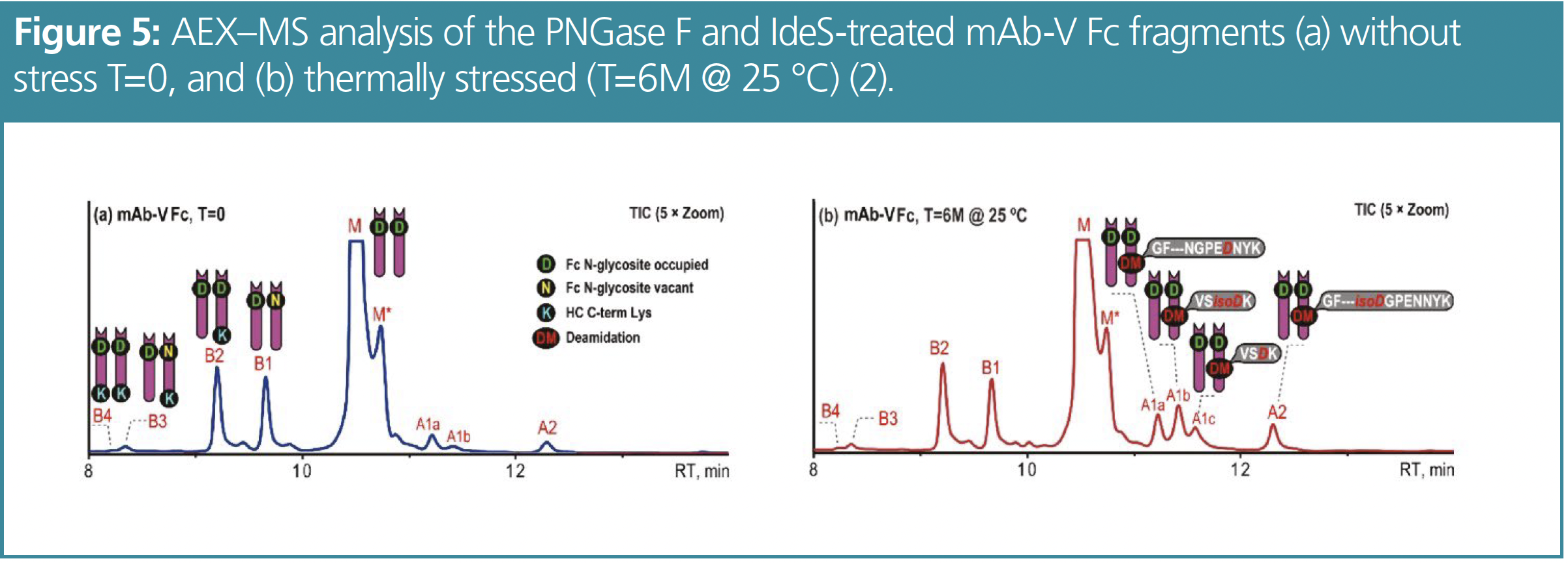
These basic variants did not change after thermal stress while an increase in peaks for the acidic variants was observed, which can be completely attributed to deamidation. Analysis of Fc fragments improved the resolution of separated deamidated variants. Four acidic peaks are resolved from the analysis of the thermally stressed mAb. Since there are only a few deamidation sites present in the IgG4 Fc region, it is likely that site-specific deamidations can be separated by AEX–MS. Possible deamidation sites are NG at VVSVLTVLHQDWLNGK; NK at VSNK; NG and NN at GFYPSDIAVEWESNGQPENNYK. Therefore, the fractions of the deamidated variants were identified by peptide mapping. Fraction A1a mainly contains the GFYPSDIAVEWESNGQPEDNYK peptide while A2 mainly contains the GFYPSDIAVEWES(isoD)GQPENNYK peptide. It is also noticeable that A1b and A1c both contain the deamidated VSNK peptide, but A1b mainly contains the isoAsp form, whereas A1c primarily contains the Asp form. It is striking that this AEX–MS method is capable of separating site-specific deamidation products at the Fc level, even at isoform resolution, so that these attributes can be monitored without the need for peptide mapping. This saves time and reduces sources of error such as deamidation artefacts that can occur in peptide mapping.
Conclusions
The AEX–MS method described is very suitable for characterising charge heterogeneity in IgG4-based mAbs. Compared to CEX–MS, AEX–MS shows overall better separation of these mAbs with moderate pI and provides additional information. The resolution of the glycosylated variants can be further improved by PNGase F-mediated deglycosylation. AEX–MS methods are suitable for the Fc critical quality attribute monitoring of IgG4-based mAbs, while CEX remains the better option for F(ab’)2 subunit analysis. With this AEX–MS method the Fc critical quality attributes can be monitored without peptide mapping, saving time, reducing errors and, even more important, providing reproducible results.
References
(1) Monoclonal Antibodies (mABs). World Health Organization. https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/monoclonal-antibodies (accessed 01/15/2023).
(2) Kinch, M. S.; Kraft, Z.; Schwartz, T. Monoclonal Antibodies: Trends in Therapeutic Success and Commercial Focus. Drug Discovery Today 2023, 28 (1), 103415. DOI: 10.1016/j.drudis.2022.103415
(3) Goyon, A.; Excoffier, M.; Janin-Bussat, M.- C.; Bobaly, B.; Fekete, S.; Guillarme, D.; Beck, A. Determination of Isoelectric Points and Relative Charge Variants of 23 Therapeutic Monoclonal Antibodies. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2017, 1065–1066, 119–128. DOI: 10.1016/j.jchromb.2017.09.033
(4) Yan, Y.; Liu, A. P.; Wang, S.; Daly, T. J.; Li, N. Ultrasensitive Characterization of Charge Heterogeneity of Therapeutic Monoclonal Antibodies Using Strong Cation Exchange Chromatography Coupled to Native Mass Spectrometry. Anal. Chem. 2018, 90 (21), 13013–13020. DOI: 10.1021/acs.analchem.8b03773
(5) Leblanc, Y.; Bihoreau, N.; Chevreux, G. Characterization of Human Serum Albumin Isoforms by Ion Exchange Chromatography Coupled On-Line to Native Mass Spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2018, 1095, 87–93. DOI: 10.1016/j.jchromb.2018.07.014
(6) Füssl, F.; Criscuolo, A.; Cook, K.; Scheffler, K.; Bones, J. Cracking Proteoform Complexity of Ovalbumin with Anion-Exchange Chromatography–High-Resolution Mass Spectrometry under Native Conditions. J. Proteome Res. 2019, 18 (10), 3689–3702. DOI: 10.1021/acs.jproteome.9b00375
(7) Liu, A. P.; Yan, Y.; Wang, S.; Li, N. Coupling Anion Exchange Chromatography with Native Mass Spectrometry for Charge Heterogeneity Characterization of Monoclonal Antibodies. Anal. Chem. 2022, 94 (16), 6355–6362. DOI: 10.1021/acs.analchem.2c00707
Ann Marie Rojahn studied chemistry and biotechnology in combination with an apprenticeship as a chemical laboratory assistant at the University of Applied Sciences in Krefeld, Germany. After receiving a bachelor’s degree, she pursued a master’s programme in chemistry at the University of Düsseldorf, Germany, with a focus on organic chemistry. Since 2019, she has worked for YMC Europe in Dinslaken as a product specialist in the analytical chromatography team.
Daniel Eßer studied chemistry at the University of Applied Sciences Bonn Rhein-Sieg, in Rheinbach, Germany, with a focus on pharmaceutical and analytical chemistry. He received his PhD in pharmaceutical and medicinal chemistry at the University of Düsseldorf, Germany. During his postdoc at the Institute of Pharmaceutical and Medicinal Chemistry of the University of Düsseldorf, he established a nanoLC–MS system. In 2013 he joined YMC Europe in Dinslaken, Germany, as a product specialist for analytical chromatography. Since 2017, he has been responsible for YMC’s analytical (U)HPLC column portfolio as the product manager for analytical chromatography. Email: d.esser@ymc.eu

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.