A NanoLC-MS Primer for Expression Proteomics, Part I
LCGC North America
The first of a two-part series addresses HPLC pumps, sample introduction systems, and columns for nanoLC-MS.
Expression proteomic studies seek to identify proteins from biological sources whose biosynthesis is quantitatively affected by the metabolic state of an organism. Proteins that are up- or down-regulated in response to a perturbation, such as disease, can be used as markers for diagnosis and monitoring or as potential targets for therapeutic intervention. The most widely used approach for determining changes in protein expression is "bottom-up" proteomics. In this approach, proteins in a bodily fluid or tissue extract are separated and identified by liquid chromatography–mass spectrometry (LC–MS). There are two strategies for bottom-up proteomics. In the first, intact proteins are first separated using two-dimensional (2D) gel electrophoresis. Protein spots on the 2D gel are subjected to proteolytic digestion, and the resultant peptides are used to identify the protein by LC–MS. In the second strategy, proteins in the bodily fluid or tissue extract are digested, and the complex peptide mixture is separated by multidimensional liquid chromatography coupled to MS for identification of all the proteins present in the original sample. This procedure forms the basis of the so-called multidimensional protein identification technology, or MuDPIT (1). In both strategies, the final step employs reversed-phase high performance liquid chromatography (HPLC) coupled via electrospray ionization to an ion trap or hybrid (for example, quadrupole time-of-flight) mass analyzer to generate MS–MS fragmentation patterns for protein identification.
In both of these approaches, the key requirement is attaining at least femtomole sensitivity at the MS detector. This arises from the small amount of sample available, or from the need to identify proteins present at very low concentration in the sample. This sensitivity requirement makes the use of conventional chromatographic techniques impractical. Using conventional (4–5 mm i.d.) or microbore (1–2 mm i.d.) columns would entail unacceptable loss in signal due to loss of protein by adsorption or dilution, or inefficient ion transfer to the MS. To obtain useful sensitivity, the column diameter must be reduced to the capillary range, 50–100 μm. This is because, for a given sample mass, the peak volume will scale in proportion to the reduction in column cross-sectional area (see Table I). The sensitivity enhancement scales in the same ratio, so that a 75-μm column provides an increase in peak concentration of over 3700-fold compared with a conventional column for an equivalent injection mass (this is somewhat misleading, because column capacity depends on packing volume, and capillary columns will be easily overloaded compared to microbore and conventional columns). An added benefit is the enhanced ionization efficiency provided by nanospray ionization. This arises from the nanoliter-per-minute flow rates used with capillary columns. At these low flow rates, the droplet size in the spray plume is smaller and more homogeneous than in conventional electrospray (2), resulting in higher surface-to-volume ratios, more efficient desolvation, and enhanced production of gas-phase ions. Also, the nanospray emitter tip is usually positioned very close (a few millimeters) to the entrance to the MS, reducing ion trajectory and spreading of the ion plume. The combination of these effects produces an estimated improvement in sampling efficiency of 500-fold relative to conventional electrospray ionization (2).
The benefits of coupling a capillary or nanoLC column with a nanospray ionization source make this system the clear choice for protein identification in proteomics studies. The next two installments of "Directions in Discovery" will address practical considerations in assembling and operating the components of a nanoLC–MS system, based upon the authors' perspectives from developing and supplying nanoLC fittings (CM), and in applying a nanoLC system for protein identification (TW). The components of a nanoLC–MS system include the solvent delivery system, the sample introduction system, the HPLC column, the tubing and fittings used to connect these components, and, finally, the nanospray ionization system and mass spectrometer. In this first installment, we will discuss the first three of these components. The choice of the nanospray MS system will depend on performance requirements and budgetary considerations, and is beyond the scope of this discussion.

Table I: HPLC column performance characteristics versus column diameter
Solvent Delivery Systems
The optimal flow rates for columns with diameters of 50–150 μm are in the nanoliter- to microliter-per-minute range (Table I). Because the complex peptide mixtures in protein digests require gradient elution, the pumping system must not only deliver accurate and precise total flow rates in this range, but must deliver solvent gradients with good precision. A summary of commercial pumping systems for nanoLC is presented in Table II. These fall loosely into two categories. Capillary pumps can deliver flow rates in the low microliter-per-minute range, but require the installation of a splitter to reduce the flow to the submicroliter-per-minute range needed for nanoLC columns. True nanoLC pumps can deliver nanoliter-per-minute flow rates without the need for installation of a splitter, or have a splitting system integrated into the pump design. Note that several commercial nanoLC systems are able to deliver multiple binary gradients for multidimensional chromatography applications.

Table II: Commercial Pumping Systems for NanoLC
For an HPLC pump, which is to be dedicated to a nanoLC–MS system, a nanoflow pump is the obvious choice. However, if the pump is to be used for other applications as well, a pump with a wider flow rate range will be more suitable. In the case of our laboratory, we wanted the flexibility to use larger diameter columns for LC–UV applications. So in our situation, a capillary pump fitted with a flow splitter between the pump and the autosampler made better sense.
Flow splitters can range from simple and cheap to complex and expensive. The simplest is a low-volume tee fitting installed between the pump and the autosampler. A restrictor line is installed on the third leg of the tee. The split ratio and the final flow to the MS is defined by the resistance of the restrictor tubing relative to the resistance of the transfer tubing to the column, the column itself, plus the resistance of the nanospray probe and emitter. The appropriate length for the resistor tubing can be determined empirically by starting with a long length of tubing, measuring the flow at the emitter tip, then systematically cutting sections from the resistor tubing until the desired flow rate is obtained. To keep the resistor tubing to a manageable length, a small internal diameter can be used. For example, in our system we use a 50 cm X 50 μm column transfer line and a 10 cm X 75 μm column packed with 3.5-μm particles. With an input flow of 5 μL/min from the pump, a 180 cm X 20 μm resistor line produced a 1:10 split ratio with a flow of 500 nL/min to the column. A concern in using such narrow-diameter tubing is the risk of plugging, which can change the split ratio. In addition, gradual increase in column pressure with age will change the split ratio. However, we have used the same resistor for close to a year with no change in column flow or back pressure.
Another concern with the use of a simple tee splitter is the need to change resistor lines if column dimensions are changed. One answer is to keep a stable of resistor lines for different applications. Another is to use a variable splitter. This can be a "passive" splitter in which the resistance is changed manually. Alternatively, an "active" splitter can be used in which the split ratio is determined electronically. Examples of passive splitters include the fixed and adjustable flow splitters from ASI (Analytical Scientific Instruments, El Sobrante, California). The fixed flow splitter uses two fluid resistors that create the split ratio; the split ratio can be changed by using different resistor cartridges. The adjustable flow splitter uses a fixed resistor in combination with an adjustable fluid resistor (metering valve); the split ratio can be changed by means of an adjustment knob.
The ASI flow splitters are independent of changes in viscosity and pressure. Another passive splitter on the market is the micro-splitter valve available from Upchurch Scientific (Oak Harbor, Washington). The body of the valve is a tee, with an adjustable needle on the outlet that directs flow to the MS system allowing a range of split ratios without needing to change the tubing. For fine control with these splitter valves, it is important to have a source of back pressure on the waste line equal to the pressure from the tubing going to the MS system.
An example of an active flow splitter is the Mass Rate Attenuator (MRA) from Rheodyne (Rohnert Park, California). This is a high-speed six-position four-port valve that transfers an aliquot of the HPLC stream to an independent stream directed to the MS system. The advantage of this active splitting system is that it is less susceptible to plugging and is unaffected by changes in mobile phase, viscosity, or temperature. The disadvantage is the requirement for an auxiliary LC pump to deliver the MS flow stream.
No matter which splitter device is used, it is important to verify the flow rate delivered to the column. This measurement should be performed at the tip of the nanospray emitter with the nanospray voltage turned off. A convenient procedure is described by New Objective (Woburn, Massachusetts) (3). First, remove any droplets from the emitter tip using the nozzle of a canned air spray positioned about 2 cm from the tip. Immediately after droplet removal, start a stopwatch and allow a new droplet to form on the emitter tip. At the conclusion of the measurement, position a calibrated micropipette (available from New Objective or Drummond Scientific [Broomall, Pennsylvania]) close to the droplet. Carefully touch the pipette tip to the droplet so that it enters the pipette by capillary action. The flow rate can be calculated using this formula:

Sample Introduction
The ability to quickly and quantitatively introduce samples onto the column is critical to proteomics experiments, particularly in high-throughput environments such as drug discovery labs and core facilities. The sample introduction system includes the autosampler and any in-line components for sample cleanup and trapping.
Autosamplers for nanoLC–MS: The use of capillary HPLC columns operated at nanoliter-per-minute flow rates requires that autosamplers be configured to minimize delay time for transfer of sample to the column. The internal volumes in the injection valve, sampling needle, and transfer lines must be minimized. This requires the use of small-diameter (50 μm or less) capillary tubing in transfer lines, which increases the risk of plugging. To help reduce this risk, it is highly recommended to only use filtered solvents and inline filters before sensitive components of the instrument. In the autosampler, it is also desirable to be able to switch the sample loop out of the mobile phase flow path shortly after injection to reduce the time required deliver the gradient to the column (dwell time, tD).
Our laboratory develops tools for expression proteomics and biomarker discovery, and routinely identifies proteins by separation with 2-D gel electrophoresis, in-gel digestion with trypsin, followed by nanoLC–MS–MS analysis. For this application, two additional autosampler features are desirable. First, we might need to analyze a concentrated sample (an intensely-stained high abundance protein) without the risk of carryover in a successive sample. For this, the ability to automatically wash the sampling needle following injection is very useful. Second, many of the samples of high interest are dilute protein digests obtained from low-abundance proteins. In this case, it is extremely important that we sample as much of the total digest as possible (our in-gel digests are typically 15–25 μL in volume). We use an autosampler with a bottom-sensing feature so that the sampling needle is automatically positioned close to the bottom of the vial or well to minimize the volume of digest remaining after sampling.
When (as in our case) it is necessary to reduce sample waste, it is important to recall that injection of more than about 60% of the loop volume will cause loss of sample to the waste port due to laminar flow effects (4). For example, our autosampler is equipped with an 8-μL loop. To ensure that the entire injected sample is transferred to the column, we "boxcar" three consecutive 5-μL injections before starting the analytical gradient.
In a high-throughput environment where samples are analyzed around the clock with automated LC–MS systems, digests can remain in the sample queue for many hours awaiting analysis. There can be concern about the integrity of the peptides during this time, particularly if they have been prepared in dilute acid (which can favor hydrolysis). Use of an autosampler with a refrigerated sample tray or compartment minimizes sample decomposition.
Sample trapping: Direct injection of peptide digests onto an analytical HPLC column has two drawbacks. First, the digest can contain chemical or particulate materials which could contaminate or clog the column, or could contaminate the ionization source. These contaminants could include gel particulates or salts, buffers, and detergent not removed from the digest. Second, introduction of large sample volumes (for example, several microliters) at nanoliter-per-minute flow rates takes excessive amounts of time. Delivering sample to the column at elevated flow rates would reduce time, but could overpressure the column and reduce column life.
A convenient alternative to direct injection of sample is placement of a sample cleanup–trapping column in line with a switching valve. During sample loading, the digest is swept from the autosampler loop onto the trapping column at high flow rate with the column valved to a waste position. Sample introduction time can easily be reduced 10-fold with this approach, and contaminants are retained on the column (particulates) or are eluted to waste (salts, buffers, and detergents).
A simple setup for a trapping column installed across the six-port, two-position MS diverter valve is shown in Figure 1. During sample loading, flow is directed onto the trapping column, through the resistor line, and to waste at about 5 μL/min. After loading, the valve is switched to bring the trapping column in line with the analytical column. Sample is flushed onto the analytical column at 500 nL/min while the remaining flow is directed through the resistor at 4.5 μL/min.
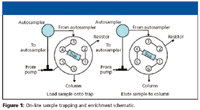
Figure 1
A disadvantage of a trapping column is loss of small hydrophilic peptides that are poorly retained on the trap. We evaluated a commercial reversed-phase trapping cartridge and observed almost a 50% loss in recovery using a commercial digest of bovine serum albumin. The poor recovery with this trap might have been due to its limited capacity. Because our interest is primarily in analysis of up- or down-regulated low-abundance proteins, we judged this to be unsatisfactory. Instead, we use direct injection at analytical flow rates, and suffer the loss in throughput. Our digestion procedure employs a destaining step, which probably removes most chemical contaminants, and we routinely centrifuge our digests to remove particulates. In our hands, column life seems not to be a problem using this sample preparation procedure with direct injection.
Vented column sample introduction
The vented column technology developed in Gygi's lab (5) eliminates the long delays of direct sample injection without the sample loss of a trapping column. In this approach, the sample enrichment column is connected directly to the analytical column across opposing arms of a PEEK cross fitting (Figure 2). A third arm of the cross contains a gold electrode connected to the high voltage source. The fourth arm contains a fritted capillary connected to a two-position six-port valve. The solvent line from the autosampler contains a splitter tee with one arm connected directly to the enrichment column and the other arm connected via the six-port valve to the resistor line of the splitter. In the sample-loading step, sample is injected into the flow path by the autosampler and the switching valve is rotated to connect the vent arm of the tee to waste. The sample is trapped on the enrichment column at a high flow rate (4 μL/min) and (if necessary) washed for several minutes to remove salts and other hydrophilic contaminants. After trapping, the valve is switched so that the vent is closed and flow is directed through both arms of the splitter tee. During analysis, flow to the column is set a 150 nL/min by the splitter.
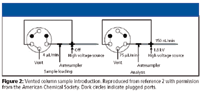
Figure 2
A commercial trapping system which has similar capabilities to the vented column approach is available from Michrom Bioresources (Auburn, California) and is shown in Figure 3. This consists of two nano-tees for connecting the trapping column to the nano analytical column and emitter tip at the MS source. The nano-tees are plumbed to a six-port two-way valve controlled by the LC–MS system. In the load position, sample is loaded at a high flow (5–50 μL/min) rate directly onto the trap column, with salts directed to waste. Once sample is loaded onto the trap, the valve is switched so that the flow is split by a resistor, and peptides are eluted from the trap to the analytical column at nanoliter-per-minute flow rates. This approach provides rapid loading and desalting of dilute samples, while minimizing the volume swept at nanoliter-per-minute flowrates, and allows conventional 1/16-in. PEEK tubing to be used for all connections from the HPLC to the trap and switching valve.
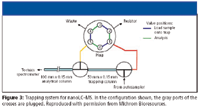
Figure 3
Capillary HPLC Columns
In the early days of nanoLC–MS, researchers packed their own columns using fused silica capillaries mounted in pressurized vessels. This practice is still common in academic research labs and core facilities where budgets are slender and student labor is affordable. In industrial laboratories, the use of commercial columns is usually preferred.
Capillary HPLC columns for nanoLC–MS are offered by several vendors. These contain porous microparticulate materials packed in polyimide-clad fused silica capillaries or in PEEKsil tubing (polymer-sheathed fused silica capillary). The vast majority of these columns are packed with 3–5 μm diameter silica particles derivatized with linear alkylhydrocarbon (for example, octyl or octadecyl) stationary phase ligands. The most popular column geometries are 75–150 μm inside diameters and 50–150 mm lengths. When operated at the same flow velocities, these provide pressures and plate numbers similar to those of conventional 4.6-mm i.d. HPLC columns packed with the same materials.
When selecting a column for LC–MS analysis of peptide digests, stationary phases bonded at high coverage on Type B silica are recommended. These have low amounts of active silanols, and can be used with acetic or formic acid as volatile mobile phase additives in place of trifluoroacetic acid (TFA). Although TFA provides best peak shape and recovery of peptides, it causes ion suppression in electrospray and nanospray ionization through formation of neutral analyte-TFA ion pairs in the gas phase. Postcolumn addition of propionic or acetic acid has been shown to reduce TFA ion suppression (the so-called "TFA fix" [6]). However, the TFA fix is not compatible with ion trap mass spectrometers due to space charge effects of the propionic acid.
In the last few years, monolithic columns have generated interest among chromatographers because of their higher porosity (which generates lower operating pressure) and their higher efficiency at elevated flow velocities. Both of these features enable their use for high throughput analysis. Monolithic columns are offered in the capillary format by two vendors. The Chromolith CapRod from E. Merck kGaA (Darmstadt, Germany) is a silica monolith with a C18 phase in a 150 mm X 100 μm column. The Monolithic Nanocolumn from Dionex (Sunnyvale, California) contains a polystyrene–divinylbenzene polymeric monolith, and has a length of 50 mm and an internal diameter of 100 μm.
As an alternative to coupling a free-standing column to the nanospray source, a packed-bed emitter can be used. For example, the PicoFrit columns from New Objective are 50 or 75 μm i.d. fused silica capillaries with 1-, 5-, or 10-cm beds packed with any of several microparticulate chromatography media. A frit integrated into the pulled emitter tip (10 or 15 μm i.d.) serves to contain the packing. Packed-bed emitters spray directly from column to inlet, minimizing extra-column band broadening.
Before installation on the nanospray source, a new column should be conditioned to saturate active sites on the packing and to eliminate any contaminants from manufacturing. We typically inject 10 pmol of a standard protein digest and run a series of blank gradients for at least 10 h before attaching the column to the MS system.
Summary
The sensitivity demands for LC–MS systems used in expression proteomics require the use of capillary HPLC columns coupled to nanospray ionization sources. Capillary columns operated at nanoliter-per-minute flow rates will require investment in new HPLC hardware or investment in time and materials to modify existing components. In this first installment of a two-part series, we have discussed solvent delivery systems, splitters, autosamplers, trapping systems, and capillary HPLC columns for nanoLC–MS. In a forthcoming installment, we will discuss tubings, fittings, and validation issues in using nanoLC–MS for proteomics.
Caitlin McEathron is research and development scientist at Upchurch Scientific (a unit of IDEX Corp.), Oak Harbor, Washington.
Tim Wehr "Directions in Discovery" editor Tim Wehr is staff scientist at Bio-Rad Laboratories, Hercules, California. Direct correspondence about this column to Direct correspondence about this column to "Directions in Discovery," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com
References
(1) A.J. Link, J. Eng, D.M. Schieltz, E. Carmack, G.J. Mize, D.R. Morris, B.M. Garvik, and J.R. Yates, Nature Biotechnol. 17, 676–682 (1999).
(2) J. Abian, A.J. Oosterkamp, and E. Gelpí, J. Mass Spectrom. 34, 244–254 (1999).
(3) Technical Note PT-7, "Setup and Measurement of Flow Rate for Online Nanobore LC–MS," New Objective, Inc. (2005).
(4) S. Bakalyar, Technical Note 5, "Achieving Accuracy and Precision with Rheodyne Manual Sample Injectors," Rheodyne. LLC (2001).
(5) L.J. Licklider, C.C. Thoreen, J. Peng, and S.P. Gygi, Anal. Chem. 74, 3076–3083 (2002).
(6) A. Apffel, S. Fischer, G. Goldberg, P.C. Goodley, and F.E. Kuhlmann, J. Chromatogr. A 712, 177–190 (1995).

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.













