How to Retain Polar and Nonpolar Compounds on the Same HPLC Stationary Phase with an Isocratic Mobile Phase
LCGC North America
Separation and retention of both polar and nonpolar compounds by the same stationary phase can be a useful approach for analyses of complex samples with a broad range of chemical properties. Typical stationary phases are designed for retention of either polar or nonpolar compounds so that multiple steps are required when designing a separation strategy. Hydride silica–based stationary phases are new materials with properties that allow for the simultaneous retention of both polar and nonpolar compounds over a range of aqueous–organic mobile phase compositions. Adjustment of the aqueous–organic ratio will determine whether polar or nonpolar compounds have greater retention.
High performance liquid chromatography (HPLC) stationary phases can be segregated by their ability to separate either polar on nonpolar compounds, that is, reversed-phase materials (C18, C8) strongly retain nonpolar solutes with polar solutes eluting at or near the void volume, and hydrophilic interaction chromatography (HILIC) and normal phase columns strongly retain polar analytes with nonpolar compounds being essentially nonretained (1). Increasingly, many analyses such as those encountered in drug discovery, proteomics, and metabolomics can be more complex with solutes encompassing a broad range of polarities. To overcome these deficiencies in column performance, more complex schemes of analysis might have to be devised to provide successfully qualitative and quantitative information about solutes with differing hydrophobicities and hydrophilicities. These approaches can include various types of sample preparation or two-dimensional chromatographic methods (2) as well as using chromatographic extremes of pH (3) and temperature (4). In most cases, such methodology can be cumbersome, time consuming, and damaging to your instrument and the HPLC column. In many instances, it would be desirable to have a stationary phase that can retain both polar and nonpolar compounds in an isocratic run so that a single separation strategy can be devised to analyze samples with a broad range of polarities.

Recently, a third type of chromatographic strategy has been developed on stationary phases utilizing silicon-hydride-based particles with a bonded phase (5–15). It is referred to as aqueous normal phase (ANP) chromatography. The principle of ANP chromatography is simple: retention behavior is analogous to that found in normal phase chromatography but the mobile phase has some water as part of the binary solvent. "Normal phase" implies that retention is greatest for polar solutes such as acids and bases. In addition, retention must increase as the amount of the nonpolar solvent in the mobile phase increases. So if the mobile phase consists of water and acetonitrile, retention increases as the amount of acetonitrile increases. Typically, in ANP chromatography, the amount of the nonpolar component in the mobile phase must be 60% or greater with the exact point of increased retention depending upon the solute and the organic component of the mobile phase.
If a stationary phase had the retention properties previously described and could only separate polar solutes, then it would be similar to a HILIC material (16–18). The term ANP is useful in distinguishing the hydride-based phases from typical HILIC phases. The hydride stationary phases also can retain nonpolar compounds by a traditional reversed-phase mechanism during the same isocratic run as described previously. Therefore, it is this dual retention capability that distinguishes the silicon-hydride material from other silica-based HPLC stationary phases. ANP chromatography is a useful term for indicating retention of ionizable–polar compounds on a silicon-hydride stationary phase also possessing reversed-phase capabilities — as opposed to a HILIC process that can separate only polar solutes.
Experimental
Instrumentation: All chromatographic experiments utilized a model 1050 HPLC system with a diode-array detector (Agilent Technologies, Wilmington, Delaware) and interfaced to a MicroMass mass spectrometer (Waters Corp., Milford, Massachusetts). The Micromass Platform LC system was equipped with an Edwards model E2M30 rotary vacuum pump (Chell Instruments, Norfolk, UK) a Micromass Platform LC model M940150DC1 atmospheric pressure chemical ionization (APCI) probe, and a computer-based data acquisition system with MassLynx (version 3.4) software (Waters). The instrument was purged with high-pressure liquid nitrogen gas (100 psi). The acquisition parameters used for all the separations were as follows: APCI pin 3.20, cone 25, skimmer 2.0, source heater 140 °C, APCI probe temperature 600 °C, gas flow 250 mL/min. 0.5% formic acid was added to the mobile phase for ionizing the test samples. All liquid chromatography–mass spectrometry (LC–MS) separations were performed using the APCI probe in the positive ion mode.
Chemicals: Metformin and glyburide were obtained from Eon Pharmaceutical (Wilson, North Carolina). HPLC-grade acetonitrile (Spectrum Chemical, Gardena, California) and methanol (VWR, West Chester, Pennsylvania) were used as organic modifiers in the mobile phase. Formic acid (Sigma-Aldrich, St. Louis, Missouri) was added to the mobile phase as the APCI reagent. Deionized water for HPLC system was obtained from a Milli-Q water purification system (Millipore, Bedford, Massachusetts).
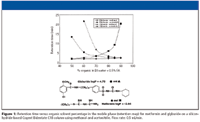
Figure 1
HPLC columns: Three columns were used in the chromatographic testing: a Cogent Bidentate C18 column (Microsolv Technology, Eatontown, New Jersey), a Luna (2) C18 column (Phenomenex, Torrance, California), and a Zorbax Extend C18 column (Agilent Technologies). All columns were obtained from the manufacturer.
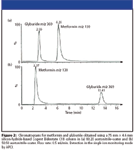
Figure 2
Results and Discussion
For methanol, only a slight increase is observed above 80% organic in the mobile phase. An interesting feature of this plot is that for acetonitrile, it is possible for the highly polar compound metformin and the nonpolar compound glyburide to be coeluted with both having reasonably good retention. This coelution would occur at 70% acetonitrile with a retention time of over 4 min. Thus, it is possible to retain both polar and nonpolar compounds on the same stationary phase by utilizing the unique properties of the silicon-hydride stationary phase. The polar compounds are retained by the ANP mechanism while the nonpolar solutes undergo typical reversed-phase behavior. It can be said that under these circumstances, the use of a silicon-hydride-based stationary phase allows for separation in two dimensions on a single column. At present, a comprehensive understanding of the simultaneous reversed-phase–aqueous normal retention mechanisms of the silicon-hydride phases has not been developed. Both chromatographic and spectroscopic investigations are underway to determine the sources of this behavior. This phenomenon could be due to the hydride surface alone, the absence of substantial amounts of adsorbed water on the surface, a magnified effect of the few remaining silanols, or some combination of these three possible properties. The stronger retention in acetonitrile is likely a polarity effect. It is less polar than methanol and as expected in a normal-phase mechanism, the least polar solvent will result in the greatest retention.
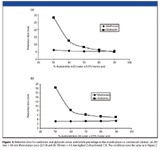
Figure 3
The actual chromatograms for retention reversal are shown in Figure 2. In Figure 2a, the conditions are such (80% acetonitrile) that the more polar solute (metformin) is eluted after the less or nonpolar compound glyburide; in this case, ANP is the dominant mechanism. By decreasing the amount of acetonitrile to 50%, it can be seen (Figure 2b) that the two compounds now are eluted in the opposite order with glyburide being more retained than metformin; that is, the dominant mechanism is typical reversed-phase interaction. These retention properties can be contrasted to the retention maps for typical reversed-phase materials that are shown in Figure 3. Two well-established C18 phases are tested under the same conditions used for the bidentate C18 column that is based upon silicon-hydride as previously described. In each case, the nonpolar compound glyburide is well retained at low acetonitrile composition in the mobile phase and not retained (at or near the void volume) as the amount of acetonitrile exceeds 80%. In contrast, the polar compound metformin is poorly retained or not retained over the whole range of acetonitrile compositions in the mobile phase. Figure 4 shows a comparison of peak shapes for the highly basic compound metformin on these three columns at constant mobile phase composition. The chromatogram on the first column (Figure 4a) demonstrates tailing that is common on many typical reversed-phase materials indicating the presence of residual silanols on the surface. In contrast, the chromatogram of metformin on the extensively endcapped second C18 column (Figure 4b) results in a highly symmetric peak. The same result is obtained on the silicon-hydride-based C18 column (Figure 4c). However, in contrast to the second column, where the peak is eluted very close to the void volume, the bidentate C18 silicon-hydride-based stationary phase has measurable retention for metformin that can be increased by using a mobile phase with a higher amount of acetonitrile.
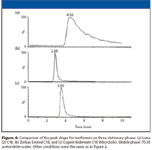
Figure 4
Other properties of silicon-hydride-based phases: Retention of glyburide in the previous example demonstrates the reversed-phase properties of the silicon-hydride-based stationary phases. To compare its properties with those of other reversed-phase materials, a standard test based upon the retention of naphthalene and acenaphthene was conducted (19). The hydrophobicity parameter for the bidentate C18 based upon this test was 0.0397. This value was within experimental error the same as the first and second C18 columns tested previously (19). Therefore, the silicon-hydride-based stationary phases have excellent reversed-phase properties along with the ANP ability not available in other hydrophobic materials.
Another crucial aspect of the ANP mechanism is its reproducibility. The retention of metformin in a 70:30 acetonitrile–water mobile phase was measured. For 10 consecutive injections, the retention time was measured as 4.08 ± 0.01 min with a RSD of 0.25%. Therefore, excellent reproducibility can be obtained in the ANP mode with these columns.
Similar ANP retention also has been demonstrated for other polar compounds (choline, acetylcholine, tobramycin, nortriptyline, imipramine, amytripyline, acetylsalicylic acid, 2-hydroxybenzoic acid, and 4-hydroxybenzoic acid) on the silicon-hydride-based stationary phases. Therefore, for many analyses, it might not be necessary to use a high-pH mobile phase to analyze polar compounds, as it would be with many bases. In the previous example, the aqueous component of the mobile phase contained 0.5% formic acid for compatibility with mass spectral analysis. The ANP at normal pH and formic acid buffered conditions are conducive to both the long-term stability of the stationary phase as well as leading to fewer instrumental problems such as pump repairs, filter replacements, and seal deterioration. In addition, the two mechanism retention characteristics of the silicon-hydride materials allow for separation of mixtures of polar and nonpolar compounds where both types of solutes can be retained and separated. Such behavior is not possible on other stationary phases unless some additives are used in the mobile phase to make polar compounds nonpolar and, hence, retained on a reversed-phase material (20) or to make nonpolar species more polar to be retained on a HILIC type stationary phase. These procedures often are not successful for every compound in the sample and usually provide only limited improvement in identifying all of the analytes. In addition, most additives are not amenable to LC–MS methods because often they are not volatile enough to be introduced into a mass spectrometer. The processes also can be accomplished by precolumn derivatization, but this adds another step to the analysis, which can be time-consuming, cumbersome, and also not successful for all compounds in the sample.
Conclusions
In summary, the example here can be duplicated with many polar–nonpolar solute pairs and illustrates one unique property of silicon-hydride-based HPLC stationary phases. Both peak shape and reproducibility of reversed-phase retained solutes and ANP retained compounds is excellent. These stationary phases are a new tool that can be used to help solve the increasingly complex analytical problems encountered in pharmaceutical, biological, and environmental samples.
Acknowledgments
The authors would like to thank MicroSolv Technology Corporation, Eatontown, New Jersey (http://www.mtc-usa.com) for donation of the columns used in this study.
References
(1) L.S. Snyder, J.J. Kirkland, and J.L. Glajch, Practical HPLC Method Development, 2nd ed. (John Wiley & Sons, Hoboken, New Jersey, 1997).
(2) P.G. Righetti, A. Castagna, B. Herbert, F. Reymond, and J.S. Rossier, Proteomics 3, 1397 (2003).
(3) J.J. Kirkland, M.A. van Straten, and H.A. Claessens, J. Chromatogr., A 797, 111 (1998).
(4) D.R. Stoll and P.W. Carr, J. Amer. Chem. Soc. 127, 5034 (2005).
(5) J.E. Sandoval and J.J. Pesek, Anal. Chem. 63, 2634 (1991).
(6) C.H. Chu, E. Jonsson, M. Auvinen, J.J. Pesek, and J.E. Sandoval, Anal. Chem. 65, 808 (1993).
(7) M.C. Montes, C. van Amen, J.J. Pesek, and J.E. Sandoval, J. Chromatogr. 688, 31 (1994).
(8) J.J. Pesek, M.T. Matyska, J.E. Sandoval, and E. Williamsen, J. Liq. Chromatogr. 19, 2843 (1996).
(9) J.J. Pesek, M.T. Matyska, E.J. Williamsen, M. Evanchic, V. Hazari, K. Konjuh, S. Takhar, and R. Tranchina, J. Chromatogr., A 786, 219 (1997).
(10) J.J. Pesek, M.T. Matyska, M. Oliva, and M. Evanchic, J. Chromatogr., A 818, 145 (1998).
(11) J.Pesek, M.T. Matyska, and P.F. Fu, Chromatographia 53, 635 (2001).
(12) J.J. Pesek, M.T. Matyska, and R.J. Yu, J. Chromatogr., A 907, 195 (2002).
(13) J.J. Pesek, M.T. Matyska, G.B. Dawson, A. Wilsdorf, P. Marc, and M. Padki, J. Chromatogr., A 986, 253 (2003).
(14) M.T. Matyska, J.J. Pesek, and X. Pan, J. Chromatogr., A 992, 57 (2003).
(15) L. Brown, B. Ciccone, J.J. Pesek, and M.T. Matyska, Am. Lab. 35 (24), 23 (2003).
(16) Y. Guo and S. Gaiki, J. Chromatogr., A 1074, 71 (2005).
(17) T. Yoshida, J. Biochem. Biophys. Methods 60, 265 (2004).
(18) B.A. Olsen, J. Chromatogr., A 913, 113 (2001).
(19) U.D. Neue, K. Van Tran, P.C. Iraneta, and B.A. Aldon, J. Sep. Sci. 26, 174 (2003).
(20) J.J. Kirkland, LCGC 14, 486 (1996).

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












