Molecular Weight Determination of LMWH SEC–MALS vs. SEC–UV-RI
Low-molecular-weight heparins (LMWHs) are obtained by fractionation or depolymerization of natural heparins. They are defined as having a mass-average molecular weight of less than 8000 and for which at least 60% of the total weight has a molecular mass less than 8000.
Low-molecular-weight heparins (LMWHs) are obtained by fractionation or depolymerization of natural heparins. They are defined as having a mass-average molecular weight of less than 8000 and for which at least 60% of the total weight has a molecular mass less than 8000.
Size-exclusion chromatography (SEC) has been the most common way of measuring the molecular weight and molecular weight distributions of LMWHs by using the two most common detection technologies: ultraviolet (UV) coupled with refractive index (RI) detection. However, these detectors embody a relative method in order to determine molecular weights, requiring calibration standards. A newer, absolute method involves the use of multi-angle light scattering (MALS), which does not require any standards. The European Pharmacopeia (EP) monograph for LMWH specifies the use of the UV-RI detection method and provides a known calibration standard. Many laboratories around the world have adopted this method.

Figure 1: Examples of UV and RI traces for an LMWH sample.
We previously developed an SEC–MALS method and found it to be very suitable for the analysis of LMWHs. We have recently adopted the UV-RI method described in the EP monograph and compared the molecular weight results generated for LMWH using each detection type. The adopted method uses an Agilent LC- 1200 series HPLC, 0.2 M Sodium Sulfate pH 5.0 mobile phase, Tosoh TSK-gel G2000 SWxl column with Tosoh TSK-gel Guard SWxl, Waters 2487 dual wavelength UV detector, and Wyatt Optilab rEX refractive index detector. For MALS analysis the UV detector was replaced with a Wyatt miniDAWN TREOS detector; all other methods aspects remained the same.
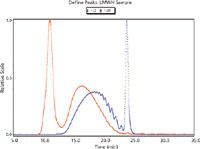
Figure 2: Examples of LS and RI traces for an LMWH sample.
The results indicated that both detection types are suitable and acceptable for the analysis of LMWHs. The molecular weight and distribution results generated using each detection type are comparable. This indicates that a SEC–MALS method could be adopted in place of the SEC–UV-RI method currently required by the EP monograph, and that it would result in less time because it obviates the need for calibration standards.
This note was graciously submitted by Lin Rao and John Beirne, Scientific Protein Laboratories LLC.
Wyatt Technology Corporation
6300 Hollister Avenue, Santa Barbara, CA 93117
tel. (805) 681-9009, fax (805) 681-0123
Website: www.wyatt.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














