Modern Techniques for the Extraction of Solid Materials — An Update
Traditional methods for the sample preparation of insoluble solid materials have represented one of the more time consuming and labour-intensive efforts in analysis. In this instalment of "Sample Prep Perspectives", Ron Majors examines modern sample preparation methods for solids that often involve increased temperature and higher pressure to speed up the extraction process. In addition, modern sample preparation methods have been automated to relieve analysts of the drudgery associated with traditional methods. Here, he reports on automated Soxhlet extraction, supercritical fluid extraction, pressurized fluid extraction–accelerated solvent extraction, and microwave-assisted extraction and updates earlier coverage.
In June 1999, LCGC N. Am. published a special supplement that focused on the sample preparation of solids.1 At that time, we covered general methods — supercritical fluid extraction (SFE), pressurized fluid extraction–accelerated solvent extraction (PFE–ASE), microwave-assisted extraction (MAE), and automated Soxhlet extraction. In the ensuing six years, these modern extraction techniques have continued to evolve. In some instances, systems have been further miniaturized and automated. A wider variety of applications has been achieved in such diverse areas as environmental, pharmaceutical, natural product, food and polymer chemistry. In this instalment of "Sample Preparation Perspectives", I will update the earlier coverage and make a note of some of the instrumental advances and a few applications that have spurred the growth of these extraction techniques. Although the literature abounds with applications examples, this review will focus on the technology. Websites of the various manufacturers, textbooks and review articles are great sources of information on applications examples.
Modern liquid–solid extraction has its roots in the shake–filter method. Basically, the shake–filter extraction method involves the intimate contact between a solid material, usually finely divided, and a solvent that has an optimum solubility for the analyte of interest and a minimum solubility for the matrix. The technique works well for porous matrices where the solvent can diffuse into the pores and extract analytes. Sometimes mechanical agitation and heating is used to speed up the extraction process. At the conclusion of the experiment, insoluble materials are removed by filtration or centrifugation. For some samples, the extract can immediately be injected into a chromatograph for further separation. More often, there is some additional sample preparation required to isolate the critical analytes from other extractables that can interfere with the chromatography step.
For more intimate contact between solvent, the adsorbed (absorbed) analyte and matrix, other sample preparation techniques might be required. Several methods that can achieve this intimate contact include homogenization, sonication, emulsification, or vortexing. For biological samples, various cell disruption techniques can be employed. All of these methods require the application of an additional external force (or forces) to the sample in suspension.
Most modern liquid–solid extraction techniques use increased temperature or pressure to increase the rate of extraction. Table 1 summarizes the influence of these parameters on analyte extraction.

Table 1: Most modern solid extraction techniques use increased temperature and pressure.
Modern Soxhlet Extraction
Soxhlet extraction is by far the most widely used method for solid sample pretreatment. In fact, it is the de facto standard with which all other extraction procedures are compared and contrasted. Soxhlet extraction is simple, effective and relatively inexpensive. Generally, it provides good analyte recovery but its traditional operation is slow and uses copious amounts of solvent. Although classical Soxhlet extractions can last as long as 18–24 h, once set up and running, the experiment requires very little user involvement. Small-volume Soxhlet systems are available, but the sample size is often dictated by the analyte concentration, the necessary mass to obtain a representative sample, and the chromatography detector sensitivity, all of which could combine to require a larger sample size.
Traditional Soxhlet extraction has been around for over 100 years, having been invented by Franz von Soxhlet in 1879. The extractor apparatus used in the traditional method is depicted in Figure 1. Typically, dry sample material is placed inside a "thimble" made from cellulose filter paper, which is loaded into the extractor. The extractor is attached to a flask containing a solvent and a condenser. The solvent is heated, causing it to evaporate. The hot solvent vapour travels up to the condenser, where it cools and drips down onto the solid material in the thimble. The chamber containing the sample slowly fills with warm solvent until, when it is almost full, it is emptied by a siphoning action back into the solvent flask. This cycle is allowed to repeat many times. During each cycle, a portion of the analyte (and perhaps some of the matrix) dissolves in the solvent.
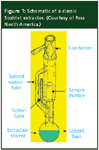
Figure 1
However, once the extracted analyte reaches the solvent heating flask, it stays there and does not participate in the extraction cycle any further. Only clean, warm solvent is used to extract the solid in the thimble, a key advantage of classical Soxhlet extraction. This procedure prevents the possibility of solvent becoming saturated with extractable material and increases the efficiency of the extraction when compared with the simple shake–filter method.
In 1974, Edward Randall made a major improvement in the Soxhlet extraction technique that reduced the extraction time dramatically.1 In his method, the sample was totally immersed in the boiling solvent. Compared with the condensed solvent in the classical Soxhlet method where the solvent temperature is below the boiling point, the Randall method was faster because analytes are more soluble in hot solvent than in cold-to-warm solvent. The apparatus used to perform the Randall adaptation is shown in Figure 2.

Figure 2
The operation of the Randall adaptation is depicted in Figure 3. First, the thimble is lowered into the boiling solvent until the appropriate extraction takes place. Then, to flush residual extract from the sample, a rinse step follows. In this second stage, the thimble is raised above the boiling solvent for a period of time until residual extract is removed from the solid material by the condensed solvent, just as is performed in the original Soxhlet experiment. Finally, the drying step removes the solvent from the solvent flask and concentrates the analyte for further processing. In this step, by closing a solvent return valve, the condensed solvent is redirected away from the sample and boiling solvent and collected in a reservoir for possible reuse or disposal. In some systems, there is a fourth step in which the sample cup is lifted from the heat source and allowed to evaporate further without the chance of sample overheating, boiling dry, or potential oxidation. The Randall approach can decrease the extraction time by as much as a factor of 10 compared with traditional Soxhlet extraction.
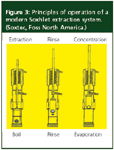
Figure 3
The Randall approach has been adopted by a number of companies with full automation including Foss-Tecator (Eden Praire, Minnesota, USA), Buchi Laboritechnik (Flawil, Switzerland) and C. Gerhardt GmbH (Bonn, Germany).
Table 2 outlines some of the main features of these systems. All of these systems can run totally unattended, offer temperature programming, solvent reclamation and numerous safety features. Most solvents can be used, although diethyl ether is not recommended for obvious reasons. Automated Soxhlet extraction is approved by the US Environmental Protection Agency (EPA) for the extraction of organic analytes from soil, sediment, sludge and waste solids (Method 3541).
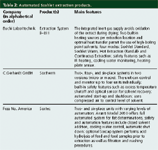
Table 2: Automated Soxhlet extraction products.
There is no doubt that because Soxhlet extraction is the de facto standard in liquid–solid extractions it will continue to be used because of its simplicity, relatively low cost, extraction efficiency and ease-of-use. With the advent of fully automated systems, much of the drudgery of classic Soxhlet extraction has been reduced.
Nevertheless, some interesting approaches to improve on the technique have been proposed. Focused microwave-assisted Soxhlet extraction could even speed the extraction time even further with certain microwave-absorbing solvents for environmental solid samples.2,3 The further application of using water as an extraction solvent makes the process much more environmentally friendly.4 When pollutants are present in aged soils, they are more difficult to extract by Soxhlet extraction than when present in spiked soils. An in situ derivatization technique was found to release pentachlorophenol more easily from sandy soil.5 Using the acetylation agents triethylamine–acetic anhydride and pyridine–acetic anhydride, the authors found that the yields were four times greater and the extraction time shorter with in situ derivatized pentachlorophenol in soil than by the traditional Soxhlet extraction of nonderivatized pentachlorophenol in the same soil.
Supercritical Fluid Extraction
When first introduced in the early 1990s, supercritical fluid extraction (SFE) was thought to be the panacea to solve all sample extraction problems for solid samples. The potential arguments were convincing — a safe, solvent-free, easily removed extractant (carbon dioxide) with a tunable solvent power that diffused like a gas and had the solvation power of a liquid. Quite a number of analytical instrument suppliers (around 12 if my memory serves me correctly) jumped on the bandwagon and instruments were introduced with full automation, variable restrictors, sample collection and many features albeit at a relatively high price tag for most. The EPA was quite keen on supporting development of SFE because there was a strong desire to decrease the use of copious amounts of solvents used in conventional Soxhlet and ultrasonic extractions. There was a vision that SFE could be the universal method to be used to extract all analytes from solid environmental matrices — soil, sand, sludge, fly ash and so on. Food analysts saw SFE as a universal extraction technology for the extraction of toxic analytes from virtually any type of food sample. Polymer chemists believed that SFE might be able to extract additives from any type of plastic, rubber, or other solid synthetic material.
Today, there are less than a handful of SFE suppliers, mainly smaller companies with dedicated instruments. As of last count, only Applied Separations (Allentown, Pennsylvania, USA), Thar Technologies (Pittsburgh, Pennsylvania, USA), LECO Corporation (St Joseph, Michigan, USA) and Jasco (Easton, Maryland, USA) still supply analytical extraction units. In 2004 Teledyne Isco (Lincoln, Nebraska, USA) discontinued the supply of SFE equipment, and the Supelco SFE-400 (Bellefonte, Pennsylvania, USA) is no longer available.
Still, even today, new applications of SFE are still being reported but from a commercial viewpoint, no new instruments or major accessories for existing instruments have appeared on the market since our original report in 1999.1 So, for commercial purposes, the analytical-scale SFE technique appears to be stagnant. Nevertheless, from a preparative and industrial-scale viewpoint, SFE is still widely used for such applications as the decaffeination of coffee and extraction of fragrances for perfumes.
Why didn't SFE achieve the potential that was purported in the early 1990s? Let us briefly examine some of the reasons where SFE went awry. First, supercritical carbon dioxide, by far the most popular SFE extractant, in its densest state (0.95 g/mL) provides the extraction power equivalent to toluene or perhaps ethyl acetate. Thus, supercritical carbon dioxide could not always quantitatively extract even moderately polar analytes, especially in aged soils or in ground, solid polymer beads. For example, the EPA Method 3561 polynuclear aromatic hydrocarbons (PAHs) that are relatively nonpolar molecules cannot be extracted from an inert matrix with pure supercritical carbon dioxide.6 To extract the larger, less volatile, fused-ring PAH aromatics such as benzo[α]pyrene and benzo[ghi]perylene, an organic modifier must be added. With this organic solvent addition, the SFE method now becomes two separate extraction procedures where the results must be combined to get the complete composition. This approach adds to the complexity and requires an SFE instrument that has an additional pump for organic modifier addition.
Indeed, most SFE methods require the addition of an organic modifier to perform satisfactorily. The amount of modifier added is sometimes small perhaps only 5–10% of the amount of carbon dioxide by volume. However, in a 30 min extraction, the amount of organic solvent could still amount to as much as 10 mL of organic solvent, which negates one of the biggest advantages of SFE: the need for little or no organic solvent. Still, the amount of organic solvent used in SFE is still reduced compared with the classical extraction methods (and even some of the other modern extraction methods).
Second, the method development in SFE was less straightforward than initially thought. The big culprits were matrix effects and effect of moisture. Even for the same analyte, a different method was often required depending upon the solid material to be extracted. These matrix effects caused the biggest problem in getting SFE methods through the EPA approval process. If a PAH was in fly ash, clay, sand, loam, peat or another soil matrix, a different procedure was required for a quantitative extraction. Environmental samples are not always so easily controlled. In addition, the EPA wanted more standardized methodology that can be placed in the hands of contract testing laboratories. The effect of moisture could often be overcome by the addition of a drying agent to the sample but required another step in the extraction process.
Similarly, sample preparation chemists attempting to use the new technique found it to be unfamiliar relative to their training and background. They had to learn new technology that was more difficult to understand and cookbook methods were not readily available for their analyte–matrix pairs. To aid in the education of the users, SFE training courses were held, symposia were arranged, books were published, application notes could be had and experienced help was available, primarily through the instrument companies who supplied SFE instrumentation. Instruments had powerful software to help in making the method development process easier. Nevertheless, the complexity of SFE method development was a hindrance to its acceptance. In contrast the more rapid acceptance of accelerated solvent extraction–pressurized fluid extraction (next section), especially by the EPA, because that technique retained the simple principles of classical liquid–solid extraction.
Third, SFE turned out to be a fairly expensive sample preparation technique. The initial price of an automated SFE unit was often more than the price of the analytical instrument that it served. First time purchasers had a hard time justifying replacing an inexpensive bank of Soxhlet extractors in their fume hood, even if the breakeven point was less than a year if one ran a reasonable number of samples per day. Liquid carbon dioxide required to perform SFE could be purchased for a reasonable cost and the amount of organic solvent required was low relative to the Soxhlet technique. Thus, the operational cost of chemicals was not prohibitive. Where the higher operating costs came into the picture was the labour content where a high level of skill was needed to successfully develop methods, in maintaining the instrument, and in troubleshooting problems and understanding the corrective actions when extractions did not go as planned. Today, the SFE instruments that are remaining on the market are not as sophisticated as they were in the 1990s so they are not quite as expensive.
Fourth, early SFE instruments fell short on performance and left the impression that the technique was difficult to use. Early round-robin interlaboratory testing gave inconsistent results.7 Instruments from different manufacturers performed differently and, therefore, methods could not be easily duplicated from lab to lab. Second-generation instruments came out with higher levels of automation such as autosamplers, automated collection devices and variable restrictors for better control over the supercritical fluid conditions but the damage had already been done.
Nevertheless, SFE has had (and continues to have) many success stories as a selective sample preparation procedure in food chemistry, pesticide residues and environmental applications. It appears to be best when the analytes are nonpolar or possess relatively low polarity and the matrices are of nonfatty composition. General reviews8,9 and entire books10–12 are dedicated to SFE.
Pressurized Fluid Extraction–Accelerated Solvent Extraction
Originally introduced by Dionex (Sunnyvale, California, USA) as ASE, the technique was generalized to PFE by the US EPA. The ASE technique was trademarked by Dionex and specific products are not generally endorsed by the EPA. Nevertheless, the PFE technique is now well accepted as an alternative to Soxhlet extraction. EPA Method 3545A, titled "Pressurized Fluid Extraction", is a recognized alternative procedure for extracting water-insoluble or slightly water-soluble organic compounds from soils, clays, sediments, sludge and waste solids. This method is applicable to the extraction of semivolatile organic compounds, organophosphorus pesticides, organochlorine pesticides, chlorinated herbicides, polychlorinated biphenyls (PCBs) and polychlorinated dibenzodioxins and dibenzofurans (PCDDs and PCDFs), which can then be analysed by a variety of chromatographic procedures. PFE, sometimes referred to as pressurized solvent extraction (PSE), uses elevated temperatures (100–180 °C) and pressures (1500–2000 psi) to achieve analyte recoveries equivalent to those from Soxhlet extraction but using less solvent and taking significantly less time. The idea is that a solvent heated above its boiling point in a closed system becomes a very potent extraction solvent. Extractions by PFE generally require only 10–20 min. Sample requirements vary but generally 1–30 g is required depending upon the sensitivity needed. Sample extraction cells can be as small as 1 mL or as large as 100 mL.
Relative to other new technologies, the EPA adopted the PFE method in less than three years while the first SFE method took nearly eight years to go through the promulgation process. Similar to other modern liquid–solid extraction technologies, PFE uses less solvent, is faster and is more automated than traditional Soxhlet extraction. The beauty of the technique is that it uses the same solvents as classical Soxhlet and sonication methods. Method development is quite easy: one chooses a solvent that is a good solvent for the analyte(s) but a poor solvent for the matrix. The instrumentation of PFE is similar to that of SFE but instead of pumping liquid carbon dioxide into the extraction chamber, conventional extraction solvents are used. Figure 4 shows a general schematic of the Dionex ASE instrument. The sample, usually in a finely ground state, is placed into an extraction cell much like the thimble in an SFE instrument. A pump transfers solvent from one or more reservoirs into the extraction cell, located in an oven. The oven heats (by conventional convection heating) the sample for a finite period of time (~10–20 min), usually determined during the method development operation. Extractions can be performed in static or dynamic modes. After the extraction is completed in the static mode, a nitrogen purge is used to transfer the extract to a collection vial. In the PFE process, the sample is contained in a large volume of solvent and additional steps are needed to concentrate the analyte of interest. If the analytes are low-to-mid polarity and the extraction solvent has high water content, passage of the extract through a reversed-phase solid-phase extraction cartridge can serve as a simple concentration device. Then a small amount of water-miscible organic solvent (acetonitrile or methanol) can elute the analyte into a vial for later injection into a high performance liquid chromatography (HPLC) or gas chromatography instrument.
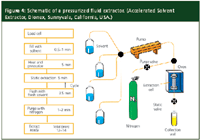
Figure 4
Although the Dionex instrument pictured in Figure 4 is serial and only one sample at a time can be extracted, the ASE 200 instrument has an autosampler that can handle as many as 24 samples for unattended operation. If higher throughput is required, Applied Separation's fast PASE system is a parallel PFE system that allows the simultaneous extraction of as many as six samples. However, the PASE does not have an autosampler, which means that it will require manual involvement every six samples.

Table 3: Extraction conditions for active ingredients in natural products.
The range of applications of PFE–ASE is as vast as that of the other classic and modern extraction techniques. Because convection heating is used in this technique, just about any solvent can be used for extraction. Table 3 provides an example of the conditions and time required for a PFE extraction of active ingredients from natural products.13 The samples were commercially available nutritional supplements, specifically hypericin from Hypericum perforatum (St John's Wort) and berberine from Hydrastis canadenis (goldenseal root). The former is used to treat kidney diseases, depression and other conditions whereas the latter is an immune system booster. Samples, in capsule form, were opened, the ground material collected and 1–3 g was loaded into the extraction cells with cellulose filter in the cell outlet. Several extraction solvents were investigated and acetonitrile, being compatible with HPLC, was used for final extraction. Using the Dionex ASE-200 equipped with 11 mL extraction cells, both sets of samples were extracted. Analysis was performed by reversed-phase HPLC using external calibration. Table 4 shows the results for four capsule extractions for each of the two supplements.13 The results were comparable to the industry standard methods for this type of extraction (Soxhlet and sonication) but were achieved much faster, used less solvent and were fully automated thereby increasing productivity of laboratory personnel.
Table 4: Results on PFEâASE extractions of natural products.
Microwave-Assisted Solvent Extraction
The use of microwaves in the laboratory was accepted long ago. Microwave digestions have been in use for over 20 years and are widely used for metal analysis in soils and other solids. Microwave-assisted extraction (sometimes referred to a microwave-accelerated solvent extraction) of organic analytes is a more recent technique. An excellent review article was included in the 1999 LCGC N. Am. supplement.14 Compared with the traditional liquid–solid extractions described earlier, the use of microwaves as a heat source has some marked differences: the extraction solvent is heated internally rather than by convective heating; the temperature in the extraction container is dictated by the solvent used for extraction and not by the set point of an external oven or heater; and as the temperature of the solvent in the closed container rises, the pressure inside rises above atmospheric pressure. The fact that the solvent itself is heated causes a more rapid response to begin the extraction process faster than with convection heating.
Of course, microwave extractions can take place in an open vessel just as you heat hot water in a cup while preparing tea. Whereas water has a high dielectric constant and readily absorbs microwave energy, organic solvents such as hydrocarbons do not absorb the microwave energy and, therefore, are not heated. For microwave extractions with these types of organic solvents via indirect heating, a microwave-absorbing device must be placed inside of the extraction vessel. These devices are usually rods or powder of a microwave-absorbing material such as carbon black coated with an inert polymeric substance (for example, PTFE) that will not contaminate the sample. Figure 5 compares the heating rate of hexane (nonmicrowave absorbing solvent), acetone (microwave absorbing solvent) and hexane with a carbon-black filled fluoropolymer heating insert, Carboflon, a product of CEM Corporation (Mathews, North Carolina, USA).
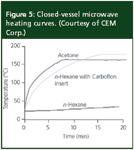
Figure 5
Figure 6 illustrates the advantage of using a microwave-absorbing insert for achieving a cleaner MAE. The experiment was to extract the hydrocarbon pollutants out of a soil sample that could be accomplished by toluene extraction alone. In Figure 6(a), the extraction solvent was a mixture of toluene and methanol. The polar methanol was required to absorb microwave energy so that the extraction could be viable for MAE. Note the noisy baseline and doublet peaks for the anthracene and phenanthrene. Also note that some polar analytes also were extracted because of the stronger polarity of this extraction solvent. Compare the cleaner chromatogram of Figure 6(b) where toluene only was used as the extract solvent combined with the use of a Weflon insert, an inert fluorocarbon microwave absorbing device of Milestone (Sorisole [BG], Italy). The anthracene and phenanthrene peak shape is better and there is an absence of unwanted polar compounds because the aromatic nonpolar hydrocarbon toluene was the only extraction solvent.
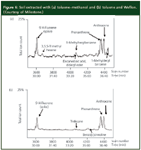
Figure 6
In microwave-assisted extraction, special nonmicrowave absorbing, inert extraction cells are required. These cells must be able to withstand high temperatures (as high as 300 °C) and high pressures (as high as 200 psi). All parts of the cells must be non-microwave absorbing and the parts touching the sample must be made of totally inert substances. The cells are constructed of glass or perfluoroalkoxy (PFA) Teflon with PFA Teflon seal covers, glass-filled polyetherimide sleeves for radial strength and polpropylene support frames with polyether ether ketone (PEEK) load disks and sealing screws for longitudinal (up–down) strength. Also, ceramic materials and fibre-reinforced PEEK are used for microwave transparent extraction cells.
Because one is dealing with organic solvents heated above their boiling points, there are many built-in safety features for commercial microwave-extraction systems. These safety features are found in both the extraction cells and in the microwave cavity of the oven. The extraction cells have safety vents in case of excessive pressure build-up. Solvent sensors are installed inside the microwave oven in case a solvent leak occurs. The extraction vessels that are commercially available from various suppliers can be temperature controlled so that the extraction temperature inside the cell can be optimally set. In some units, an infrared sensor is available for contactless temperature monitoring. In addition, because stirring is desirable to ensure intimate contact between the finely divided samples and the extraction solvent, vessels can also be stirred using a stirring bar. Oven doors can also be safety protected in case of a catastrophic internal pressure build-up in the oven itself.
Because multiple samples can be simultaneously processed in a microwave oven, the sample throughput can be very high compared with some of the other liquid–solid extraction techniques. For example, both Milestone's Ethos high throughput system and CEM MARS systems have the capability for as many as 24 or 40 extraction vessels/batch, respectively.
Recent developments in MAE have included a new approach to control the microwave energy inside of an extraction vessel as the dielectric media changes. In the new automated ExplorerPLS small-scale microwave sample prep system from CEM Corp., the single-mode self-tuning microwave cavity compensates for changes in physical properties of the sample as it changes during the application of the microwave field. The mechanical design of the cavity allows the microwave to enter the cavity wherever it wants, dictated by the sample in the cavity that draws the microwave from a series of windows cut into the circular waveguide inner wall. Originally designed as a CEM microwave synthesis system, the ExplorerPLS sample prep system for MAE uses disposable high-pressure 10 mL extraction tubes with 0.1–7 g working volume for applications for samples as small as 100 mg.
Microwave extraction is now widely accepted as an alternative to Soxhlet, PFE–ASE and other liquid–solid extraction techniques. For example, similar to the EPA Method 3545 described earlier for PFE–ASE, EPA Method 3546 is an MAE procedure for extracting water insoluble or slightly water soluble organic compounds from soils, clays, sediments, sludges and solid wastes. This method is applicable to the extraction of semivolatile organic compounds, organophosphorus pesticides, organochlorine pesticides, chlorinated herbicides, phenoxyacid herbicides, substituted phenols, PCBs and PCDDs and PCDFs. The method uses microwave energy to produce elevated temperature and pressure conditions (i.e., 100–115 °C and 50–175 psi) in a closed vessel containing the sample and organic solvent(s) to achieve analyte recoveries equivalent to those from Soxhlet extraction (Method 3540) using less solvent and taking significantly less time than the Soxhlet procedure.
Comments About the Use of Modern Liquid–Solid Extraction Procedures
With the exception of SFE, the use of the modern liquid–solid extraction procedures is a variation of a theme. They all use the same types of solvents and can accomplish the same types of applications. Each of them uses a slightly different approach in achieving the same results. For example, PFE–ASE and MAE both use high temperature and high pressure to achieve faster extractions than traditional or automated Soxhlet extraction but the results achieved are the same. In fact, most modern extraction techniques treat the traditional Soxhlet extraction as the de facto standard. Because the extraction conditions are better controlled in automated systems, the relative standard deviations can be somewhat better than the traditional methods. However, the cost of the newer automated systems is considerably higher than the equipment needed to perform traditional Soxhlet extraction. The biggest cost saving argument for the modern approaches is the reduction of solvent usage and also in the reduction in manual labour. Also, most of the systems offer a lower degree of exposure of workers to organic solvents. Whereas, with the modern liquid–solid approaches, the operation of organic solvent extractions at high pressure and high temperature requires extraordinary safety features to be built into the equipment that, in turn, also causes a purchase price increase. For a high throughput laboratory requiring daily extractions of many samples of solid materials such as soils, food samples, plastic materials and natural products, the added investment in automated systems should pay for itself in a relatively short period of time.
SFE has lost some of its lustre, with only a few companies offering this technology. However, it still has its place in niche applications and is a safe extraction technique that is considered to be environmentally friendly. The operating cost is among the lowest since the use of organic solvents with associated purchase and disposal costs is minimal.
Acknowledgments
The author would like to thank the following companies and individuals for providing information to complete the article: Greg LeBlanc, CEM Corp.; Bruce Richter, Dionex Corp.; Shirley Anderson, Foss No. America; Merrill Loechner and Robert Richter, Milestones.
References
1. E.L. Randall, J.A.O.A.C., 57(5), 1165–1198 (1974).
2. J.L. Luque-Garcia and L. de Castro,
J. Chromatogr A., 998(1–2), 21–29 (2003).
3. J.L. Luque-García et al., Chromatographia, 62(1–2), 69–74 (2005).
4. J.L. Luque-Garcia and M.D. Luque de Castro, Anal. Chem., 73(24), 5903–5908 (2001).
5. S.M. Soto-Cordoba, J. Baeza, and J. Freer, Bol. Soc. Chil. Quím, 46(2), 179–185 (2001).
6. http://www.epa.gov/sw-846/pdfs/3561.pdf
7. R. Stevenson, Amer. Lab., Oct., 36–37 (1993).
8. J.M. Levy, LCGC N. Am., 17(6S), S14–S21 (1999).
9. R.Smith, J. Chromatogr. A, 1000(1–2), 3–27 (2003).
10. L.T. Taylor, Techniques in Analytical Chemistry: Supercritical Fluid Extraction (Wiley, New York, 1996).
11. M.D. Luque De Castro, M. Valcarcel, and M.T. Tena, Analytical Supercritical Fluid Extraction (Springer, New York, 1994).
12. E.D. Ramsey, Ed., Analytical Supercritical Fluid Extraction Techniques (Kluwer Academic Publishers, Dordrecht, The Netherlands, 1998).
13. "Accelerated Solvent Extraction (ASE) of Active Ingredients from Natural Products", Application Note 335, Dionex Corp., Sunnyvale, California.
14. G. LeBlanc, LCGC N. Am., 17(6S), S30–S37 (1999).
15. R.C. Richter et al., presented at Pittcon '99, Orlando Florida, March 8, 1999.
Ronald E. Majors is business development manager, Consumables and Accessories Business Unit, Agilent Technologies, Wilmington, Delaware, USA and is a member of the Editorial Advisory Board of LCGC Europe. Direct correspondence about this column to LCGC Europe, Advanstar House, Park West, Sealand Road, Chester CH1 4RN, UK, e-mail: dhills@advanstar.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









