Modern Column Technologies for the Analytical Characterization of Biopharmaceuticals in Various Liquid Chromatographic Modes
Special Issues
Modern Column Technologies for the Analytical Characterization of Biopharmaceuticals in Various Liquid Chromatographic Modes
The recent trends in column technology for reversed-phase liquid chromatography (LC), size-exclusion chromatography (SEC), ion-exchange chromatography (IEX), and hydrophobic interaction chromatography (HIC) for analysis of biopharmaceuticals at the protein level is critically discussed.
Photo Credit: Jorg Greuel/Getty Images
Therapeutic proteins are large and heterogeneous molecules subjected to a variety of enzymatic and chemical modifications during expression, purification, and long-term storage. These changes include several possible modifications, such as oxidation, deamidation, glycosylation, aggregation, misfolding, or adsorption, leading to a potential loss of therapeutic efficacy or unwanted immune reactions. Regulatory bodies require a detailed characterization (for example, verifying primary structure and appropriate post-translational modifications, secondary and tertiary structure), lot-to-lot and batch-to-batch comparisons, stability studies, impurity profiling, glycoprofiling, determination of related proteins and excipients as well as determination of protein aggregates. For this purpose, a single analytical technique is generally not sufficient, and a variety of orthogonal methods are required to fully describe such a complex sample. Today, one of the most widely used analytical techniques for therapeutic protein characterization is liquid chromatography (LC). This is probably a direct result of the remarkable developments of the past few years, which have enabled a new level of chromatographic performance. These developments include ultrahigh-pressure LC (UHPLC), columns packed with wide-pore superficially porous particles (SPPs), and organic monolith columns, which have allowed a dramatic increase in separation efficiency, even with large intact biomolecules. This article will review the possibilities and trends of current state-of-the-art LC column technology applied for different modes of chromatography for the characterization of therapeutic proteins.
Hydrophobic Interaction Chromatography
Hydrophobic interaction chromatography (HIC) has been historically used for protein purification; more recently, the two main application fields have been in the determination of the drug-to-antibody ratio (DAR) of antibody-drug conjugates (ADCs) and in monitoring post-translational modifications of monoclonal antibodies (mAbs). In HIC, proteins are retained and separated on the basis of their hydrophobicity as a result of the van der Waals forces between the hydrophobic ligands of the stationary phase and the non-polar regions of proteins (1). The binding of proteins to a hydrophobic surface is affected by a number of factors including the type of ligand, the ligand density on the solid support, the backbone material of the stationary phase, the hydrophobic nature of the protein, and the type of salt added to the mobile phase. During the separation, a negative salt gradient (typically from 2–3 M to 0 M) is applied under aqueous mobile phase at around pH 6.8–7.0. The structural damage to the biomolecules is therefore minimal and its biological activity is maintained (2). Analytical-scale HIC columns are based either on silica or polymer particles. Both porous and non-porous particles are available. Highly cross-linked non-porous poly(styrene–divinylbenzene) (PS/DVB) and polymethacrylate-based particles are frequently used in protein separations as a result of their advantageous mass transfer properties (the main contribution to the band broadening of large biomolecules, namely trans-particle mass transfer resistance is negligible). Table 1 summarizes the most widely used and the latest HIC columns applied for mAb and ADC separations.
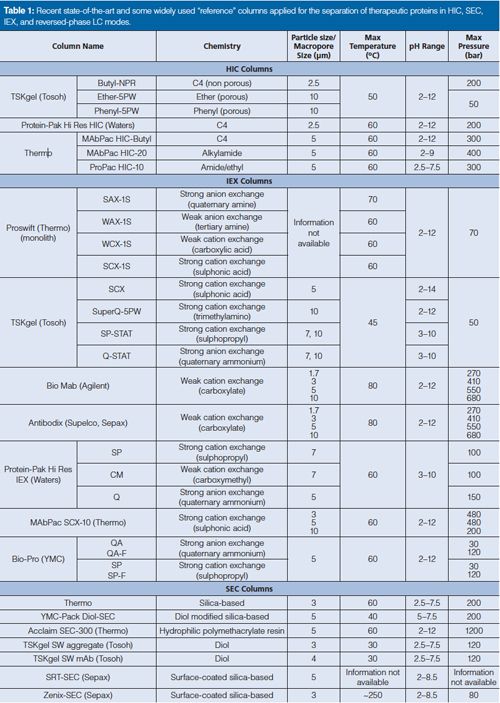
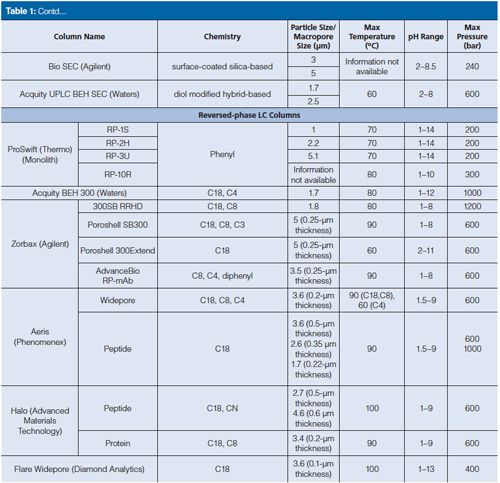
Table 1: Recent state-of-the-art and some widely used "reference" columns applied for the separation of therapeutic proteins in HIC, IEX, and reversed-phase LC modes.
These materials can now withstand pressure drops of up to 100–400 bar. Columns are typically packed with 10-, 7-, 5-, 3-, and even 2.5-µm particles. Column diameters between 2 and 8 mm are available but 4.6-mm i.d. columns are the most widely used in current HIC applications. It is worth mentioning that there is a need for 150 x 2.1 mm column formats, which is often applied for the analysis of proteins in modern chromatographic practice. HIC allows both the characterization of the distribution of drug-linked species and the determination of average DAR of ADCs (3). Conjugation of the drug-linker to the antibody increases the hydrophobicity; therefore HIC appears as a suitable tool to separate the different DAR species. A good example of the HIC profile of a native IgG1 ADC is shown in Figure 1 (4).
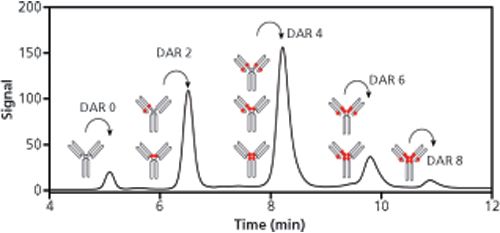
Figure 1: HIC separation of an ADC for the determination of drug-to-antibody ratio (DAR). Adapted and reproduced with permission from
Analytical Chemistry
84
, Lan N. Le, Jamie M.R. Moore, Jun Ouyang,
et al
.,
Profiling Antibody Drug Conjugate Positional Isomers: A System-of-Equations Approach
, 7479–7486 (2012) © American Chemical Society. Recently an off-line mass spectrometric (MS) detection was applied for the characterization of Brentuximab-vedotin. Each individual HIC peak was collected, buffer exchanged, and analyzed by native MS (5). HIC was also successfully applied for monitoring various post-translational modifications, including proteolytic fragments, domain misfolding, tryptophan oxidation, and aspartic acid isomerization in therapeutic mAbs (6).
Ion-Exchange Chromatography
Ion-exchange chromatography (IEX) is widely used for the characterization of therapeutic proteins and can be considered as a reference marker and powerful technique for the qualitative and quantitative evaluation of charge heterogeneity. Among the different IEX modes, cation-exchange chromatography (CEX) is the most widely used for protein characterization (7). Two modes of elution are often applied for protein characterization, namely the (i) salt-gradient and the (ii) pH-gradient. In salt-gradient mode, solutes are eluted in order of increasing binding charge, which correlates more or less with the isoelectric point (pI) and equilibrium constant. In this case, the mobile phase pH is kept constant, while the ionic strength is continuously increased. In pH-gradient mode, the ionic strength is kept constant and the pH is varied during the gradient programme. This mode of elution is often referred to as chromatofocusing. Regarding the stationary phase, there are two main aspects that need to be considered for successful IEX separation: (i) the strength of interaction and associated retention (strong or weak ion exchanger) and (ii) the achievable peak widths (efficiency) (8). Both cation and anion exchangers can be classified as either weak or strong exchangers. Weak cation exchangers are comprised of a weak acid that gradually loses its charge as the pH decreases (for example, carboxymethyl groups), while strong cation exchangers are comprised of a strong acid that is able to sustain its charge over a wide pH range (for example, sulphopropyl groups). On the other hand, strong anion exchangers contain quaternary amine functional groups, while weak anion exchanger possesses diethylaminoethane (DEAE) groups. As a rule of thumb, it is preferable to begin the method development with a strong exchanger, to ensure that a broad pH range can be worked on. Strong exchangers are also useful if the maximum resolution occurs at an extreme pH. However, silica-based ion exchangers can only be operated in a restricted pH range. In contrast, polymeric ion exchangers can be used over a wide pH range. Commercially available IEX columns are based on silica or polymer particles but organic-polymeric monoliths are also available. Both porous and non-porous particles are available but for large molecules, which possess low diffusivity, non-porous materials are clearly preferred. Highly cross-linked non-porous PS/DVB materials are most frequently used in protein separations because of their high pH stability (2 ≤ pH ≤ 12). These materials can now withstand pressure drop of up to 500–600 bar in some cases. Columns packed with 10-, 5-, or 3-µm non-porous particles are often used, but sub-2-µm materials are also available to perform UHPLC separations (see Table 1). Suitable peak capacity can be attained with large biomolecules on those columns within a reasonable analysis time (e.g. 15–20 min). However, some limitations can be expected in terms of loading capacity and retention when applying non-porous materials. A recent study systematically compared the latest state-of-the-art cation exchanger columns applied for the characterization of therapeutic mAbs in pH- and salt-gradient modes (8). Figure 2 shows an example of the separation of four intact antibody charge variants using a 100 x 4.6 mm, 5-µm strong cation polymeric exchanger column packed with non-porous particles and a 20-min long gradient (9)
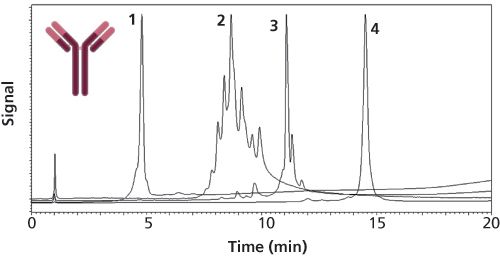
Figure 2: IEX separation of four intact mAbs (natalizumab [1], cetuximab [2], adalimumab [3], and denosumab [4]). Adapted and reproduced with permission from
Journal of Pharmaceutical and Biomedical Analysis
111
, Szabolcs Fekete, Alain Beck, and Davy Guillarme,
Characterization of cation exchanger stationary phases applied for the separations of therapeutic monoclonal antibodies
, 169–176 (2015) © Elsevier.
Size-Exclusion Chromatography
Size-exclusion chromatography (SEC) is a powerful technique for the qualitative and quantitative evaluation of protein aggregates. The main advantage of SEC is the mild mobile phase conditions that permit the characterization of proteins with minimal impact on the conformational structure and local environment. SEC separates biomolecules according to their hydrodynamic radius. The stationary phase consists of spherical porous particles with a carefully controlled pore size, through which the biomolecules diffuse based on their molecular size difference using an aqueous buffer as the mobile phase. Basically, SEC is an entropically controlled separation process in which molecules are separated on the basis of molecular size differences (filtering) rather than by their chemical properties (10). Therefore, retention factor (thermodynamic) in SEC is different from other chromatographic modes. Here, the thermodynamic retention factor is the fraction of the intraparticle pore volume that is accessible to the analyte (11). Since no retention occurs in SEC, large pore volumes (high porosity) are required to ensure appropriate resolution. Generally, this large pore volume is provided by long- and wide-bore columns. In routine SEC applications, a 30-cm column length with internal diameters (I.D.) of 7.8-, 8.0-, or 10-mm is generally employed. These SEC columns are referred to as standard bore columns. Now, several vendors offer narrow bore columns with 4.6-mm i.d. and 15-cm length that are packed with very efficient, small particles of ~ 3 µm. Similar separation power can be attained using these columns as with 5-µm particles in 30-cm standard bore columns, but the analysis time can be reduced by a factor of 3 to 4 (12). There are mainly two types of SEC packing materials: (i) silica, with or without surface modification, and (ii) cross-linked polymeric packings, which possess non-polar (hydrophobic), hydrophilic, or ionic character (10). The most common silica packing consists of chemically bonded 1,2-propanediol functional groups that provide a hydrophilic surface. This stationary phase blocks or reacts with many of the acidic silanol groups allowing the surface to be neutralized. Bare silica is also a suitable packing material for non-aqueous polar or non-polar organic mobile phases; however, it is not recommended with aqueous mobile phases because of the presence of active silanol sites. The latest type of silica-related packing is an ethylene-bridged hybrid inorganic-organic (BEH) material that is currently available at particle sizes of 1.7 µm - the first sub-2-µm SEC packing - and 2.5 µm (13). Compared to regular silica packings, BEH particles have improved chemical stability as well as reduced silanol activity. The 1.7-µm BEH material can be operated at up to 600 bar. There have been a number of different hydrophilic cross-linked packings developed for the SEC of biopolymers. Most of these packings are proprietary hydroxylated derivatives of cross-linked polymethacrylates (10). Unusual polymeric packings for aqueous SEC include sulphonated cross-linked polystyrene, polydivinylbenzene derivatized with glucose or anion exchange groups, a polyamide polymer, and high-performance, crossed-linked agarose (10).
Today, columns for aqueous and non-aqueous SEC applications with pore sizes of 125 to 900 Å are commercially available (14). Very fast separations of peptides, myoglobin, and insulin aggregates have been demonstrated with 1.7-µm SEC columns (15). These columns were also applied for the characterization of recombinant mAbs (13). Applying 1.7- and 2.5-µm particles in SEC has opened up a new level of separation performance, but it should be kept in mind that on very fine particles, the separation quality is improved at the cost of pressure (and frictional heating temperature gradients). Therefore, there is a risk of creating on-column aggregates when analyzing sensitive proteins under high pressure (> 200 bar) conditions (13).
Reversed-Phased Liquid Chromatography
In reversed-phased liquid chromatography (LC), the solute retention is predominantly mediated through hydrophobic interactions between the non-polar amino acid residues of the proteins and the bonded n-alkyl ligands of the stationary phase. Compared to the HIC mode, the reversed-phase LC mobile phase typically consists of water, acetonitrile or methanol, and 0.1-0.2% trifluoroacetic acid or formic acid. The separation mechanism is based on a combination of solvophobic and electrostatic interactions, the latter being governed by the interaction of TFA with basic side chains of a few amino acids (that is, arginine, lysine, and histidine) and the N-terminus as well as ionic interactions between the positive charges at the surface of the protein and the negatively charged residual silanols (16). The efficiency of reversed-phase LC is always superior to other chromatographic modes and its superior robustness makes it well suited for use in a routine environment (17). Current reversed-phase LC stationary phases used for proteins analysis can be classified as silica-based particulate materials and organic monoliths. The pore size of particulate phases is an important factor that must be considered. For the analysis of peptides and small proteins, a pore size between 100–200 Å may be acceptable. However, porous materials with pore sizes of more than 200 Å are mandatory for the separation of larger proteins or mAbs fragments because the solute molecular diameter must be approximately one-tenth the size of the pore diameter to avoid the restricted diffusion of the solute and to allow the total surface area of the sorbent material to be accessible. An average pore size between 250–300 Å is often mentioned as the reference value for protein separations, but recently it was shown that 400 Å particles completely eliminated restricted diffusion effects for molecules up to about 500 kDa. The two main trends today in reversed-phase LC analysis of therapeutic proteins are the use of (i) fully porous small particles (FPPs) (sub-2-µm) and (ii) superficially porous particles (SPPs), which possess particle sizes between 3- and 4 µm. Columns packed with FPPs have constraints in separation speed and efficiency because of limitations in the stationary phase mass transfer, which results from the relatively long diffusion times required for proteins to cross the porous structure. Therefore, Horváth first applied the concept of SPPs in the late 1960s (18,19). They were initially intended for the analysis of macromolecules such as peptides and proteins. SPPs are made of a solid, non-porous silica core surrounded by a porous shell layer. They have similar properties to the fully porous materials conventionally used in HPLC. The rationale behind this concept was to improve column efficiency by shortening the diffusion path that molecules must travel, in addition to improving their mass transfer kinetics. It was recently shown that columns packed with wide-pore 3.6-µm and 3.4-µm SPPs showed significant gain in analysis time and peak capacity compared to FPPs for intact protein analysis (20,21). These wide-pore SPPs are now available with C4, C8, and C18 chemistries and can be operated up to 600 bar. Figure 3 shows an example of fast separation of heavy-chain (Hc) and light-chain (Lc) variants of an IgG1 mAb performed on a wide-pore C4 SPP column.
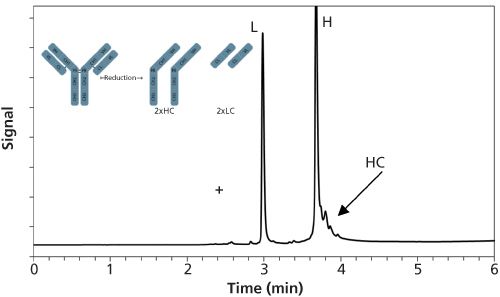
Figure 3: Reversed-phase LC analysis of reduced IgG1 mAb. Unpublished results from the authors’ laboratory.
In another study, efficiency and analysis times of 1.7-µm SPPs and FPPs were compared for peptides and moderate size intact proteins (22). This study suggests a two-fold increase in terms of achievable peak capacity and analysis time for large proteins when using SPPs compared to FPPs of the same size. For the separation of peptides and moderate size proteins, a 160 Å SP packing was also introduced (23,24). Recently 1.3- and 1.6-µm SPPs were also applied for peptide mapping of mAb samples (25,26). By combining long columns (200–300 mm) with extended analysis time, peak capacity around 1000 can be reached with 1.3-µm SPPs for 0.5–2 kDa peptides. An alternative, carbon-nanodiamond based C18 superficially porous material was recently introduced (27). The core of this material is a carbonized poly(divinylbenzene) particle with a diameter of approximately 3.4-μm. Poly(allylamine)-nanodiamond hetero-layers are deposited onto the surface of the carbonized core by a modified layer-by-layer method. The resulting core-shell is synthesized to a shell thickness of ca. 0.1 μm and a finished particle size of 3.6 μm. This superficially porous carbon-based material was successfully applied for real life protein separations. Another interesting alternative to SPPs was proposed by Hayes
et al.
(28). The so-called sphere-on-sphere (SOS) approach provides a simple and fast one-pot synthesis in which the thickness, porosity, and chemical substituents of the shell can be controlled by using the appropriate reagents and conditions (29). SOS particles have been shown to be microporous with a pore diameter of less than 2 nm. However, while the surface of the material might not exhibit significant porosity, when packed into a HPLC column the spaces between surface nanospheres provide superficial macroporosity. It has been proposed that for large molecules, larger pores as well as reduction of the shell thickness can be advantageous, because of the shorter diffusion distance and greater access to the surface area of the material (30). SOS particles were demonstrated to have similar chromatographic performance compared to commercial SPP materials (28). Figure 4 shows the separation of reduced ADC (Brentuximab-Vedotin) fragments on a column packed with SOS particles.
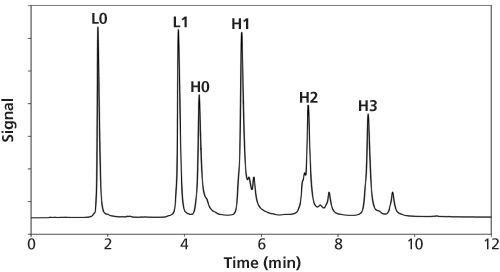
Figure 4: Reversed-phase LC separation of reduced ADC (Brentuximab-Vedotin) fragments. Unpublished results from the authors’ laboratory. As an alternative to particle-based stationary phase formats for the LC separations of proteins, organic polymer-based monoliths offer some advantages, including high permeability and rapid mass transfer (31). Polymeric monolithic stationary phases have shown great potential for the reversed-phase LC separations of large biomolecules, including intact proteins, oligonucleotides, and peptides. With this material, the mass transfer is mainly driven by convection, rather than diffusion, because of the absence of mesopores (32). The fact that the solvent is forced to pass through the macropores of the polymer because of pressure leads to faster convective mass transfer compared to the slow diffusion process into the stagnant pore liquid that is present in porous beads packed columns. As a result of their open channel structure, monoliths generally possess a high permeability, allowing the application of elevated flow rates at moderate back pressure. It was previously demonstrated that polymeric stationary phases led to superior performance over silica-based materials in the reversed phase analysis of very large proteins (MW >50 kDa) (33).
Conclusion
There is always a need to use several chromatographic methods to draw reliable conclusions regarding the quality of biopharmaceuticals. IEX, SEC, and HIC are historical techniques and are still used in any laboratory dealing with the analytical characterization of mAbs or ADCs. These techniques were known to offer poor resolving power, which is why the stationary phases employed in IEX, SEC, and HIC have strongly evolved over the last few years, in terms of chemistries, dimensions, and chemical stability. The most important improvements for protein analysis were brought to reversed-phase LC materials. In the past, this technique has rarely been used for biopharmaceutical characterization. However, because this is the only chromatographic approach directly compatible with MS, providers have improved and developed their existing materials. The performance that can be achieved today with columns packed with wide pore sub-2-µm fully porous or sub-4-µm superficially porous are highly competitive, and even if the selectivity of reversed-phase LC is still limited for separating charge or size variants of proteins, this is (at least partially) compensated by the high kinetic performance generated by modern reversed-phase LC columns.
Acknowledgements
The authors acknowledge Alain Beck (Pierre Fabre, Saint-Julien Genevois, France) for providing mAb and ADC samples, and Stephanie Schuster (Advanced Materials Technology) and Tony Edge and Richard Hayes (Thermo Fisher Scientific) for providing stationary phases. Davy Guillarme wishes to thank the Swiss National Science Foundation for support through a fellowship to Szabolcs Fekete (31003A_159494).
References
(1) C.J.V. Oss, R.J. Good, and M.K. Chaudhury, J. Chromatogr.376, 111–119 (1986).
(2) J.A. Querioz, C.T. Tomaz, and J.M.S. Cabral, J. Biotech.87, 143–159 (2001).
(3) L.N. Lee, J.M.R. Moore, J. Ouyang, X. Chen, M.D.H. Nguyen, and W.J. Galush, Anal. Chem.84, 7479–7486 (2013).
(4) Lan N. Le, Jamie M.R. Moore, Jun Ouyang, et al., Analytical Chemistry84, 7479–7486 (2012).
(5) F. Debaene, A. Boeuf, E. Wagner-Rousset, O. Colas, D. Ayoub, N. Corvaïa, A. Van Dorsselaer, A. Beck, and S. Cianférani, Anal. Chem.86, 10674–10683 (2001).
(6) M. Haverick, S. Mengisen, M. Shameem, and A. Ambrogelly, mAbs6, 852–858 (2001).
(7) S. Fekete, A. Beck, and D. Guillarme, Am. Pharm. Rev.18, 59–63 (2015).
(8) S. Fekete, A. Beck, and D. Guillarme, J. Pharm. Biomed. Anal.111, 169–176 (2015).
(9) Szabolcs Fekete, Alain Beck, and Davy Guillarme, Journal of Pharmaceutical and Biomedical Analysis111, 169–176 (2015).
(10) H.G. Barth and G.D. Saunders, LCGC North Am.30, 544–563 (2012).
(11) P. Hong, S. Koza, and E.S.P. Bouvier, J. Liq. Chrom. Rel. Techn.35, 2923–2950 (2012).
(12) S. Fekete, A. Beck, J.L. Veuthey, and D. Guillarme, J. Pharm. Biomed. Anal.101, 161–173 (2014).
(13) S. Fekete, K. Ganzler, and D. Guillarme, J. Pharm. Biomed. Anal.78–79, 141–149 (2013).
(14) E. Gazal, Can size exclusion chromatography (SEC) be done on sub-3 um particles?, presented at the 17th annual meeting of the Israel Analytical Chemistry Society, Tel Aviv, Israel (2014).
(15) S. M. Koza, P. Hong, K.J. Fountain, Advantages of ultra performance liquid chromatography using 125 Å pore size, sub-2 µm particles for the analysis of peptides and small proteins, poster presented at Medimmune, Rockville, MD, USA (2012).
(16) S. Fekete, J.L. Veuthey, and D. Guillarme, J. Pharm. Biomed. Anal. 69, 9–27 (2012).
(17) K. Sandra, I. Vandenheede, and P. Sandra, J. Chromatogr. A1335, 81–103 (2014).
(18) C. Horvath, B.A. Preiss, and S.R. Lipsky, Anal. Chem. 39, 1422–1428 (1967).
(19) C. Horvath and S.R. Lipsky, J. Chromatogr. Sci.7, 109–116 (1969).
(20) S. Fekete, R. Berky, J. Fekete, J.L. Veuthey, and D. Guillarme, J. Chromatogr. A1236, 177–188 (2012).
(21) S.A. Schuster, B.M. Wagner, B.E. Boyes, and J.J. Kirkland, J. Chromatogr. A1315, 118–126 (2013).
(22) S. Fekete, K. Ganzler, and J. Fekete, J. Pharm. Biomed. Anal.54, 482–490 (2011).
(23) F. Gritti and G. Guiochon, J. Chromatogr. A1218, 907–921 (2011).
(24) S.A. Schuster, B.M. Wagner, B.E. Boyes, and J.J. Kirkland, J. Chromatogr. Sci.48, 566–571 (2010).
(25) S. Fekete and D. Guillarme, J. Chromatogr. A1320, 86–95 (2013).
(26) B. Bobály, D. Guillarme, and S. Fekete, J. Sep. Sci.37, 189–197 (2014).
(27) B. Bobály, D. Guillarme, and S. Fekete, J. Pharm. Biomed. Anal.104, 130–136 (2015).
(28) R. Hayes, P. Myers, T. Edge, H. Zhang, Analyst139, 5674–5677 (2014).
(29) A. Ahmed, W. Abdelmagid, H. Ritchie, P. Myers, and H. Zhankg, J. Chromatogr. A1270, 194–203 (2012).
(30) L.E. Blue and J.W. Jorgenson, J. Chromatogr. A1218, 7989–7995 (2011).
(31) C. Viklund, F. Svec, J.M.J. Fréchet, and K. Irgum, Chem. Mater.8, 744–750 (1996).
(32) M. Petro, F. Svec, I. Gitsov, and J.M.J. Fréchet, Anal. Chem.68, 315–321 (1996).
(33) S. Fekete, J.-L. Veuthey, S. Eeltink, and D. Guillarme, Anal. Bioanal. Chem. 405, 3137–3151 (2013).
Szabolcs Fekete
holds a PhD degree in analytical chemistry from the Technical University of Budapest, Hungary. He worked at the Chemical Works of Gedeon Richter Plc at the analytical R&D department for 10 years. Since 2011, he has worked at the University of Geneva in Switzerland. He has contributed 70 journal articles and authored book chapters. His main interests include liquid chromatography, column technology, pharmaceutical, and protein analysis.
Jean-Luc Veuthey
is professor at the School of Pharmaceutical Sciences, University of Geneva, Switzerland. He has also acted as President of the School of Pharmaceutical Sciences, Vice-Dean of the Faculty of Sciences, and finally Vice-Rector of the University of Geneva. His research domains include development of separation techniques in pharmaceutical sciences, and, more precisely, the study of the impact of sample preparation procedures in the analytical process; fundamental studies in liquid and supercritical chromatography; separation techniques coupled with mass spectrometry; and analysis of drugs and drugs of abuse in different matrices. He has published more than 300 articles in peer-reviewed journals.
Davy Guillarme
holds a PhD degree in analytical chemistry from the University of Lyon, France. He is senior lecturer at the University of Geneva in Switzerland. He has authored 140 journal articles related to pharmaceutical analysis. His expertise includes HPLC, UHPLC, HILIC, LC–MS, SFC, and analysis of proteins and mAbs. He is an editorial advisory board member of several journals including
Journal of Chromatography A
,
Journal of Separation Science
, and
LCGC North America
.

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











