Harnessing the Benefits of Mass Spectrometry for In-depth Antibody Drug Conjugates Analytical Characterization
Special Issues
Recent progress in high-resolution mass spectrometry (HRMS) and liquid chromatography–mass spectrometry (LC–MS) methods for the structural characterization of brentuximab vedotin and trastuzumab emtansine are presented.
Antibody drug conjugates (ADCs) are a fast growing class of empowered anti-cancer biopharmaceuticals. Also known as immunoconjugates, ADCs are composed of cytotoxic drugs covalently attached via a conditionally stable linker to monoclonal antibodies (mAbs) highly specific for tumour-associated antigens. Compared to naked mAbs, ADCs have an increased level of complexity as the heterogeneity of conjugation cumulates with the inherent microvariability of the immunoglobulins (IgGs). This article highlights recent progress in high-resolution mass spectrometry (HRMS) and liquid chromatography–mass spectrometry (LC–MS) methods for the structural characterization of brentuximab vedotin and trastuzumab emtansine, two FDA and EMA approved ADCs directed against haematological and solid tumours, respectively.
Photo Credit: Evgeny Kuklev/Getty Images

Monoclonal antibodies (mAbs) and their related products are the fastest growing class of human therapeutics (1). Sixty IgGs and derivatives (antibody drug conjugates [ADCs], radio-immunoconjugates, bispecific antibodies, Fab fragments, and Fc-fusion proteins and peptides) have been approved for use in various arenas such as cancers, inflammatory diseases, and, more recently, for the treatment of high cholesterol. In oncology, however, the first generation mAbs often lack efficiency or face resistance as a result of upregulation of HER2 downstream signalling pathways in the case of trastuzumab for example (1). ADCs are emerging as an important subclass of armed immunoglobulins (2), with two approved first-in-class drugs, namely brentuximab vedotin (Adcetris, Seattle Genetics/Takeda) and trastuzumab emtansine (Kadcyla, Genentech/Roche), now on the market. Importantly, 50 more ADCs are now investigated in clinical trials in many different types of cancers. They are the result of numerous technological improvements based on a better understanding of structure-function relationships, thanks in no small part to state-of-the-art mass spectrometry (MS), which will be discussed below (4).
The Anatomy of Antibody Drug Conjugates
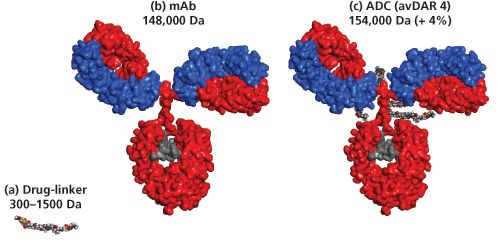
ADCs (around 154 kDa) are constructed from three components: a mAb (around 148 kDa) that is specific to a tumour antigen, a highly potent cytotoxic agent, and a chemical linker (0.3 to 1.5 kDa) that enables covalent attachment of an average of four cytotoxic payloads to the mAb (Figure 1). For most ADCs, the primary sites used for protein-directed conjugation are the sulphydryl groups of the interâchain cysteine residues or amino groups of lysine residues of the mAbs (5). Alternatively, glycans or engineered amino acids or tags can be used as attachment sites to yield more homogeneous site-specific conjugates, which often results in an improved therapeutic index (6).
The ADC Analytical Toolbox
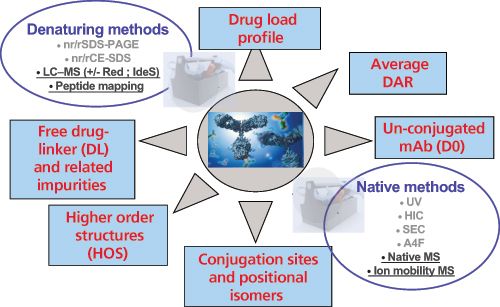
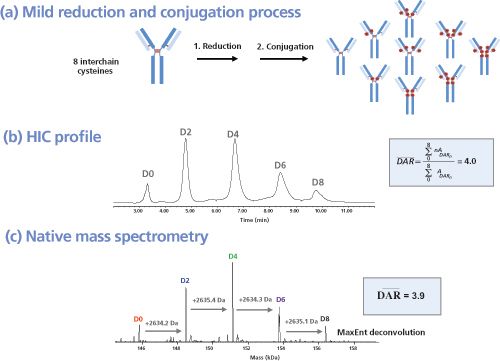
As highlighted in Figure 2, a combination of native and denaturing methods are mandatory to gain structural insights of IgG hinge cystein-linked drug conjugates. In analogy to brentuximab vedotin, nearly two-thirds of the immunoconjugates currently in clinical trials are produced through partial reduction of the four interâchain disulphide bonds of IgG1 antibodies (chimeric, humanized, or human), followed by alkylation with a preformed drug-linker maleimide activated species (Figure 3[a]). This process results in conjugates with a distribution of 0, 2, 4, 6 or 8, drugs incorporated per antibody and an average drug to antibody ratio (DAR) of 4 drugs/mAb (7). This is routinely controlled by hydrophobic interaction chromatography (HIC) under non denaturing conditions (8) (Figure 3[b]). In an orthogonal way, these structures are confirmed both by reversed-phase high performance liquid chromatography (HPLC) under reducing conditions and capillary electrophoresis sodium dodecyl sulphate (CE–SDS) under both nonâreducing and reducing conditions (9). Ultimately, MS methods are used for structural assessment (10); this includes hydrogen-deuterium exchange MS (HDX-MS) for interrogating the higher-order structure of ADCs, as recently reported by ValliereâDouglass
et al
. (11).
For the mAb moiety, dozens of microvariants have been identified and reported in the literature such as glycoforms, charge, cysteine-related, oxidized, size, and low level point mutation variants (12). Conjugation of payloads to mAbs increases the structural complexity of the resulting molecule (13), which triggers the need for improved characterization methods for the analysis of drug loading and distribution, average DAR, size and charge variants, un-conjugated drug-linker, and ADC biophysical properties (14). As for antibodies, ADCs are analyzed at different levels (top, middle, and bottom) as illustrated below for brentuximab vedotin and trastuzumab emtansine, two first-in-class and gold standards for cysteine and lysine conjugated ADCs, respectively.
Brentuximab Vedotin Characterization Under Native Conditions (Top Level, 148 to 160 kDa)
Using native desalting conditions, Valliere-Douglass
et al.
reported the expected mass measurement of the intact bivalent structure of the hinge-cysteine linked ADCs (15), which would normally decompose as a consequence of the denaturing chromatographic conditions typically used for liquid chromatography−mass spectrometry (LC−MS). The mass of the desalted ADC was determined using standard desolvation and ionization conditions. Successful intact mass measurement of IgG1 mAbs conjugated with maleimidocaproyâmonomethyl Auristatin F (mcMMAF) and valineâcitrulline-monomethyl Auristatin E (vcMMAE) at inter-chain cysteine residues were reported. This method was also used to detect the changing drug load distribution over time from a set of
in vivo
samples (16). As an alternative, Chen
et al.
reported the use of limited enzymatic digestion with a cysteine protease for vcMMAE characterization by native MS with an improvement of low abundance D6 and D8 species (17). Tandem native MS on brentuximab vedotin was successfully used by Dyachenko
et al.
to show that drug conjugation takes place non homogeneously to cysteine residues both on the light and heavy chains (18).
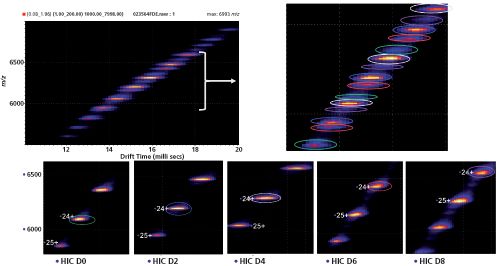
In addition, Debaene
et al.
recently reported on the combination of native MS to ion mobility MS (IM–MS) for ADC characterization (19). As a proof of principle, they highlighted the benefits of high-resolution native MS (Figure 3[c]) and native IM–MS for the determination of the drug load profile, naked antibody content, and average DAR of brentuximab vedotin (Figure 4). The analytical potential of native MS and IM–MS was compared to HIC, the gold standard for ADC quality control. The benefits of high-resolution native MS were demonstrated for drug distribution and average DAR determination along with improved mass accuracies (<30 ppm in routine analysis). The main advantage of using native MS for exact mass measurements of ADCs with interâchain cysteinylâlinked drugs lies in its ability to detect nonâcovalent associations of light and heavy chains that cannot be directly analyzed by classic denaturing LC–MS methods. Interestingly, heterogeneity of drug loading on mAbs was uniquely evidenced by differences in drift times. Collisional cross sections were measured for each payload species and affirmed slight conformational changes induced by drug conjugation. Finally, a semi-quantitative interpretation of IM–MS data was presented that allowed the average DAR and DAR distribution to be directly extrapolated. Both native MS and IM–MS experiments were in agreement with results obtained from HIC. For full proof of principle, HIC fractions were collected and analyzed by native MS and IM–MS, allowing HIC limitations, for example in the case of highly hydrophobic payloads, to be circumvented (Figure 4).
Brentuximab Vedotin Subunit Characterization under Denaturing and Reducing Conditions (Middle Level, 23 to 54 kDa)
For hinge cysteine-conjugated ADCs, most of the inter-chain disulphide bridges are no longer present but are replaced with the linker-drugs during conjugation and the ADC is held together through non-covalent hydrophobic interactions (Figure 3[a]). Reversed-phase HPLC offers an orthogonal method for drug load profiling and for calculating the average DAR. In analogy to HIC, reversed-phase HPLC is also based on hydrophobicity differences but the use of organic solvent and a small amount of organic acid instead of salt is disruptive for the intact cysteine-linked ADC. When analyzed directly, the ADC cannot withstand the highly denaturing conditions and will dissociate into antibody fragments. Treatment of the cysteineâlinked ADC with dithiothreitol (DTT) or (tris(2-carboxyethyl)phosphine) (TCEP) fully reduces the remaining inter-chain disulphides and yields six species: light chain with 0 and 1 drug attached (L0 and L1), and heavy chain with 0, 1, 2, or 3 drugs attached (H0, H1, H2, and H3). These species are stable in the denaturing organic environment and can be well resolved on a reversed-phase column such as PLRP-S. The weighted average DAR is obtained by integration of the light and heavy chain peaks and calculation of the percentage peak area; the assigned drug load for each peak must also be taken into account (20).
Brentuximab Vedotin Subunit Characterization under Denaturing Conditions after IdeS Digestion and Reduction (Middle Level, 23 to 28 kDa)
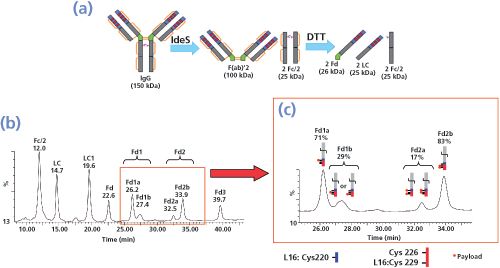
The immunoglobulin-degrading enzyme of Streptococcus pyogenes (IdeS, Fabricator, Genovis) is becoming increasingly popular for the fast characterization of antibodies by MS, including correct sequence assessment, antibody Fab and Fc glyco-profiling, biosimilar comparability studies, and Fc-fusion protein studies. IdeS specifically cleaves immunoglobulin G and related products under its hinge domain. As shown by WagnerâRousset
et al.
, IdeS is also a powerful enzyme for fast characterization (drug loading, distribution, and average DAR) of ADCs and antibodyâfluorophore conjugates (AFC). It also allows glyco-profiling to be performed, which is an important quality control method for ADCs that retain the effector functions of the naked parent antibody (ADCC). Janin-Bussat
et al.
recently confirmed that IdeS digestion of brentuximab vedotin followed by reduction significantly improved the peak separation as shown by LC–MS analysis (Figure 5[a]) (20). In addition to the seven expected majors peaks (Fc/2, L0, L1, Fd0, Fd1, Fd2, and Fd3) two minor satellite peaks were also present close to Fd1 and Fd2 with similar masses (Figure 5[b]). They were interpreted as payload positional isomers by Le
et al.
using the abundance of the different DAR species (HIC data), combined with both reversedâphase HPLC and CE–SDS data (21). The ultimate confirmation can be obtained by peptide mapping based, for example, on endoprotease Lys-C digestion of isolated peaks and LC–MS–MS analysis. This will be discussed below.
Brentuximab Vedotin Positional Isomers Characterization (Bottom Level, 0.3 to 7 kDa)
To confirm that peaks with the same masses are payload positional isomers, peaks Fd1a, Fd1b, Fd2a, and Fd2b (Figure 5[b]) were enriched by reversed-phase HPLC collection. The characterization of each fraction of interest was achieved after endoprotease Lys-C digestion and LC–MS analysis. Peptide mapping of ADCs with hydrophobic drugs linked to their native cysteine residues by LC–MS analysis is challenging because the conjugated peptides are not very soluble in aqueous buffers. This is especially true for peptides with two or more conjugated drugs. As an improvement on the peptide mapping protocol of brentuximab vedotin (LysâC digestion), all of the steps including enzymatic digestion were optimized to maintain the hydrophobic drug-loaded peptides in solution by the addition of solvents (10% acetonitrile added to the sample before digestion and 40% isopropanol after digestion). The data confirmed that the drug was linked, as expected, to the inter-chain cysteines of the heavy and light chains. Furthermore, LC–MS–MS confirmed the payload positional isomers. It was unambiguously demonstrated that the drug was linked preferentially to the heavy chain (HC) L15 peptide on Cys220 when only one drug was bound to the HC. In contrast, when two drugs were linked to the HC, they were preferentially bound to the HC L16 peptide on Cys 226 and Cys229. For further analysis and following ad hoc sample preparation as reported by Lebert
et al.
(22), a similar chromatographic separation of ADC peptides combined with MS analysis can also be applied to pharmacokinetic studies for characterization of the ADCs drugâloaded peptides distribution after their
in vivo
administration.
Trastuzumab Emtansine (Top Level, Deglycosylated, 145 to 154 kDa)
As demonstrated for cysteine conjugates, HIC and native MS are the key techniques for studying drug distribution, the naked antibody content, and the average DAR. For lysine ADC conjugates on the other hand, which are not amenable to HIC because of their higher heterogeneity, denaturing MS and UV–vis spectroscopy are the most powerful approaches.
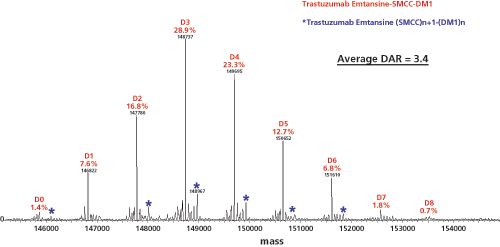
In analogy to trastuzumab emtansine, the ADC huN901–DM1 contains an average of three to four DM1 drug molecules per huN901 IgG1 antibody molecule (same DL). The composition of the mixture was determined by MS of the deglycosylated conjugate by Wang
et al.
(23). Deglycosylation eliminates the complexity in the MS spectrum caused by the heterogeneity in the glycosylation of the CHO cellâderived huN901 antibody. Samples were analyzed using size-exclusion chromatography (SEC) coupled on-line with electrospray ionization (ESI) time-of-flight (TOF)-MS to avoid salt interference with protein ionization (24). A representative ESI-TOF-MS spectrum of lysine conjugated trastuzumab emtansine is shown in Figure 6. More recently, Marcoux
et al.
reported the use of native MS and ion mobility (IM–MS) for the characterization of trastuzumab emtansine, also known as T-DM1 or Kadcyla (25). This lysine conjugate has recently been approved for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer, and combines the anti-HER2 antibody trastuzumab (Herceptin) with the cytotoxic microtubule-inhibiting maytansine derivative, DM1. Native MS combined with high-resolution measurements and charge reduction is beneficial for the accurate values it provides of the average DAR and the drug load profiles. The use of spectral deconvolution was investigated in detail. In addition, the use of native IM–MS to directly determine DAR distribution profiles and average DAR values, as well as a molecular modelling investigation of positional isomers in T-DM1, was reported.
Conclusion
The development and optimization of ADCs is increasingly being driven by a need to improve its analytical and bioanalytical characterization by assessing the main ADC quality attributes: drug load distribution, amount of naked antibody, and average DAR. These needs have been recently fulfilled by a number of cutting-edge MS methods and optimized workflows used at different levels. At the top level, native MS and native IM–MS is successfully used in addition to HIC, the reference method for quality control of interâchain cysteinyl-linked ADCs. At the middle level, reduced or IdeS-digested ADCs are analyzed by LC–MS, which is used as an orthogonal method to gain structural insights on ADC subunits. In addition, the use of IdeS allows the analysis of the Fc/2 that has been separated from the Fd fragment and the light chain. As a result, the full glycoâprofiling and demonstration of the absence of additional conjugation are easily achieved. At the bottom level, improved ADC LC–MS peptide mapping methods used to characterize the drug-loaded peptides and to identify positional isomers at cysteine residues have been developed. All the steps of the method including enzymatic digestion have been optimized to maintain the hydrophobic drugâloaded peptides in solution by the addition of solvents.
Acknowledgements
The authors acknowledge F. Debaene and J. Marcoux (LSMBO, Strasbourg, France) and E. WagnerâRousset, M.C. JaninâBussat, O. Colas, M. Excoffier, L. MorelâChevillet, C. Klinguer-Hamour, and T. Champion (CIPF, St-Julien en Genevois, France) for their contribution to the development of new ADC characterization methods.
References
(1) A. Beck, T. Wurch, C. Bailly, and N. Corvaia,
Nat. Rev. Immunol.
10
, 345–352 (2010). (2) A. Beck, J.F. Haeuw, T. Wurch, L. Goetsch, C. Bailly, and N. Corvaia,
Discov. Med.
10
, 329–339 (2010). (3) A. Beck and J.M. Reichert,
MAbs
6
, 15–17 (2014). (4) A. Beck, P. Senter, and R. Chari,
MAbs
3
, 331–337 (2011). (5) A. Beck, J. Lambert, M. Sun, and K. Lin,
MAbs
4
, 637–647 (2012). (6) C. Klinguer-Hamour, P. Strop, D.K. Shah, L. Ducry, A. Xu, and A. Beck,
MAbs
6
, 18–29 (2014). (7) P.D. Senter and E.L. Sievers,
Nat. Biotechnol.
30
, 631–637 (2012). (8) M.M. Sun, K.S. Beam, C.G. Cerveny, K.J. Hamblett, R.S. Blackmore, M.Y. Torgov, F.G. Handley, N.C. Ihle, P.D. Senter, and S.C. Alley,
Bioconjug. Chem.
16
, 1282–1290 (2005). (9) L.N. Le, J.M. Moore, J. Ouyang, X. Chen, M.D. Nguyen, and W.J. Galush,
Anal. Chem.
84
, 7479–7486 (2012). (10) J.F. Valliere-Douglass, S.M. Hengel, and L.Y.Pan,
Mol. Pharm.
12
, 1774–1783 (2015). (11) L.Y. Pan, O. Salas-Solano, and J.F. Valliere-Douglass,
Anal. Chem.
86
, 2657–2664 (2014). (12) A. Beck, E. Wagner-Rousset, D. Ayoub, A. Van Dorsselaer, and S. Sanglier-Cianferan,
Anal. Chem.
85
, 715–736 (2013). (13) A. Beck,
MAbs
6
(1), 30–33 (2014). (14) A. Wakankar, Y. Chen, Y. Gokarn, and F.S. Jacobson,
MAbs
3
, 161–172 (2011). (15) J.F. Valliere-Douglass, W.A. McFee, and O.Salas-Solano,
Anal. Chem.
84
, 2843–2849 (2012). (16) S.M. Hengel, R. Sanderson, J. Valliere-Douglass, N. Nicholas, C. Leiske, and S.C. Alley,
Anal. Chem.
86
, 3420–3425 (2014). (17) J. Chen, S. Yin, Y. Wu, and J. Ouyang,
Anal. Chem.
85
, 1699–1704 (2013). (18) A. Dyachenko, G. Wang, M. Belov, A. Makarov, R.N. de Jong, E.T. van den Bremer, P.W. Parren, and A.J. Heck,
Anal. Chem.
87
, 6095–6102 (2015). (19) F. Debaene, A. Boeuf, E. Wagner-Rousset, O. Colas, D. Ayoub, N. Corvaia, A. Van Dorsselaer, A. Beck, and S. Cianferani,
Anal. Chem.
86
, 10674–10683 (2014). (20) M.C. Janin-Bussat, M. Dillenbourg, N. Corvaia, A. Beck, and C. Klinguer-Hamour,
J. Chromatogr. B
981–982
, 9–13 (2015). (21) E. Wagner-Rousset, M.C. Janin-Bussat, O. Colas, M. Excoffier, D. Ayoub, J.F. Haeuw, I. Rilatt, M. Perez, N. Corvaia, and A. Beck,
MAbs
6
, 173–184 (2014). (22) D. Lebert, G. Picard, C. Beau-Larvor, L. Troncy, C. Lacheny, B. Maynadier, W. Low, N. Mouz, V. Brun, C. Klinguer-Hamour, M. Jaquinod, and A. Beck,
Bioanalysis
7
, 1237–1251 (2015). (23) A.C. Lazar, L. Wang, W.A. Blattler, G. Amphlett, J.M. Lambert, and W. Zhang,
Rapid Commun. Mass Spectrom.
19
, 1806–1814 (2005). (24) L. Wang, G. Amphlett, W.A. Blattler, J.M. Lambert, and W. Zhang,
Protein Sci.
14
, 2436–2446 (2005). (25) J. Marcoux, T. Champion, O. Colas, E. Wagner-Rousset, N. Corvaia, A. Van Dorsselaer, A. Beck, and S. Cianferani,
Protein Sci.
24
, 1210–1223 (2015).
Dr. Alain Beck
is Senior Director, Antibody/ADC Physico-Chemistry and member of the board of directors of the Centre d’Immunologie Pierre-Fabre. He has contributed to the R&D of anticancer mAbs (in collaboration with Merck and Abbvie), vaccines, and peptides. He is associate editor of mAbs, an inventor of 16 patents, author of more than 140 publications and reports, and he has contributed to more than 200 meetings.
Dr. Sarah Cianférani
is CNRS Research Director and Director of the BioOrganic Mass Spectrometry Laboratory (LSMBO) at the IPHC, University of Strasbourg, France. Her research is focused on developments and applications of advanced native MS, IM–MS, and HDX-MS methods for biological non-covalent complex characterization.

Evaluating the Accuracy of Mass Spectrometry Spectral Databases
May 12th 2025Mass spectrometry (MS) can be effective in identifying unknown compounds, though this can be complicated if spectra is outside of known databases. Researchers aimed to test MS databases using electron–ionization (EI)–MS.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









