MISER LC–MS Analysis of Teas, Soft Drinks, and Energy Drinks
LCGC North America
In this study, we describe a simple and rapid liquid chromatography–mass spectrometry (LC–MS) method for the evaluation of caffeine, taurine, and aspartame in teas, soft drinks, and energy drinks using high performance liquid chromatography (HPLC) coupled with electrospray ionization (ESI)-MS detection and multiple injections in a single experimental run (MISER) analysis.
In this study, we describe a simple and rapid liquid chromatography–mass spectrometry (LC–MS) method for the evaluation of caffeine, taurine, and aspartame in teas, soft drinks, and energy drinks using high performance liquid chromatography (HPLC) coupled with electrospray ionization (ESI)-MS detection and multiple injections in a single experimental run (MISER) analysis. The misergrams obtained from the injection of one sample per minute allows the convenient visualization of the outcomes of multiple analyses, allowing rapid comparison of analyte levels among a variety of products and brands.
The technique of liquid chromatography–mass spectrometry (LC–MS) is evolving into a workhorse technology used across many industries and disciplines for routine chemical analysis and problem solving (1–7). Recent trends in miniaturization and cost reduction suggest an opportunity for even more widespread adoption of LC–MS beyond the traditional laboratory setting (8–13). Consequently, studies that familiarize students and other newcomers with the practical aspects of LC–MS are becoming increasingly important. While mastery of LC–MS may require many years of training and experience, a working knowledge can be picked up in a short time, especially when simplified versions of the technique are used.

Multiple injections in a single experimental run (MISER) (14) is a simplified form of LC–MS that is well suited to fast analysis of the presence of a given component within multiple samples (14–19). Minimal chromatographic separation is used, allowing fast analysis while separating the component of interest from substances that could potentially interfere with MS detection. The injection of multiple samples in the same experimental run allows a straightforward interpretation of the MS detector response (or "misergram") providing ready interpretation without the need for difficult and time-consuming peak integration, data processing, and graphing.
We previously investigated the application of MISER LC–MS to a study of the level of capsaicin in chili peppers and hot sauces, finding the experimental approach to be easily grasped by students (19). We now report an investigation into the use of MISER LC–MS for the analysis of caffeine, aspartame, taurine, and other components within teas, soft drinks, and energy drinks, with the hope that this approach may be utilized by students and others interested in rapidly developing a familiarity with LC–MS and high-throughput analysis.
Experimental
Instrumentation
Reversed-phase high performance liquid chromatography (HPLC) experiments were performed on an Agilent 1100 system. The Agilent stack comprised a G1312A binary pump, a G1367A WPALS autosampler, a G1315B diode-array detector, and a 6120 quadrupole LC–MS detector with electrospray ionization in the positive mode. The system was controlled by Chemstation software, with the flow injection analysis (FIA) mode enabled.
Chemicals, Reagents, and Stationary Phases
Acetonitrile (HPLC grade) was purchased from Fisher Scientific. Formic acid (HCOOH), ammonium formate (NH4HCO2), caffeine, aspartame, taurine, theanine, vitamin C, vitamin B5, and sucralose were purchased from Sigma–Aldrich. Ultrapure water was obtained from a Milli-Q Gradient A10 water purification system from Millipore. The 50 mm × 4.6 mm, 2.5-µm XBridge Phenyl column was purchased from Waters Corporation.
Preparation of Buffer Solutions
Solutions containing 2 mM ammonium formate in water (pH 3.5) and 2 mM ammonium formate in acetonitrile (pH 3.5): 12.6 g ammonium formate and 7.9 mL formic acid were dissolved in 1 L of Millipore water. A 100-fold dilution of this stock solution was performed in either pure water or a 90:10 acetonitrile–water mixture to prepare the 2 mM solutions.
Sample Preparation
Tea - Time Course
A single Lipton All Natural tea bag was placed in 8 oz (approximately 240 mL) of boiling water, and the bag was submersed using a kitchen thermocouple. A 2-mL sample was taken at t = 0 s (before tea bag addition), 30 s, 60 s, and every minute thereafter up to 8 min of total steeping time. (Note: The tea bag was not intentionally moved during the course of the experiment.)
Tea - General
Brewed tea samples were achieved by placing the noted amount of tea in a Williams–Sonoma Open Kitchen Tea Ball, or a single tea bag where noted, and placing the ball or bag in 8 oz of boiling water for 5 min. The temperature was recorded at the start and end of the steeping, and a 2-mL sample was taken for analysis at the end of brewing. (Note: The tea ball [or bag] was not intentionally moved during the course of the experiment.) The observed average brewing temperatures were Tinit = 97 °C, to Tfinal = 80 °C.
Store Beverages
Samples of various beverages, including Fuze iced tea, were obtained from a local supermarket by high school interns and directly analyzed without treatment. Authentic standards of caffeine, aspartame, and taurine were prepared by serial dilution and analyzed along with the beverage samples.
HPLC–MS Conditions
HPLC separations were carried out on a 50 mm × 4.6 mm, 2.5-µm XBridge Phenyl column by isocratic elution at a flow rate of 1 mL/min. The LC eluents were 60% solvent A (2 mM ammonium formate in water, pH 3.5) and 40% solvent B (2 mM ammonium formate in 90:10 acetonitrile–water, pH 3.5). The column and samples were maintained at a temperature of 25 °C. The misergrams were obtained from continuous sample injections (1 µL) every 1 min. The positive ion electrospray ionization (ESI) parameters were as follows: skimmer, 45 V; desolvation gas, nitrogen; temperature, 350 °C; and flow rate, 12 L/min. The nebulizer was adjusted to 35 psig, and the fragmentor and the capillary voltages were adjusted to 150 and 2500 V, respectively. G1969-85000 ESI-L Low Concentration Tuning Mix (Agilent Technologies) was used for tuning and calibration of the mass spectrometer.
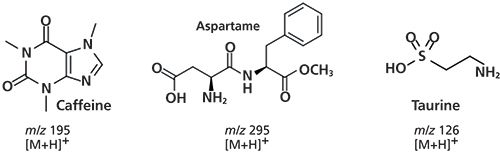
Figure 1: Structures of caffeine, aspartame, and taurine.
Ion Suppression and Enhancement Between Target Analytes
Six sets of samples were prepared at 0.25 mg/mL. Set 1 contains aspartame and caffeine; set 2, caffeine; set 3, caffeine and taurine; set 4, aspartame; set 5, aspartame and taurine; and set 6, taurine. All six samples were analyzed per duplicated using the standardized MISER LC–ESI(+)-MS method (as described above). In addition, a faster MISER LC–ESI(+)-MS method was evaluated for comparison: Column: Zorbax Stablebond SB-C18 (20 mm × 3.0 mm, 1.8 µm, Agilent); temperature: 40 °C; detection: selected ion monitoring (SIM) ESI-MS(+) at m/z = 195, 295, and 265 amu; sample: 0.6-µL injection of each component solution in acetonitrile–water; flow rate: 1 mL/min; isocratic mobile phase: 10% eluent A: 2 mM ammonim formate in water (pH 3.5) and 90% eluent B: 2 mM ammonium formate in 10:90 water–acetonitrile (pH 3.5). Time between injections was 22 s. The extent of ion suppression and ion enhancement effects are clearly visualized in the corresponding misergrams.
Results and Discussion
Students learning elementary principles in chemistry can find ready subject matter for study in the modern world. In addition to the practical advantages of sample availability, convenient disposal, and reduced requirements for personal protective equipment, the study of materials that can be found in a local supermarket provides a quick connection with the everyday world, reinforcing the important message that chemistry is omnipresent in the modern world. The analysis of caffeine in beverages is a popular laboratory experiment with a proven ability to engage students (20,21). Bier and Grabowski (22) have even implemented virtual MS approaches. In this study, we apply the MISER LC–MS technique to the measurement of caffeine and other substances in teas, soft drinks, and energy drinks collected at local supermarkets.
Samples of commercial teas were brewed before analysis (see the experimental section). A time-course study of the appearance of caffeine in brewed tea upon the addition of water is shown in Figure 2a. Sample aliquots were removed at designated timepoints and analyzed using MISER LC–MS with a sample injection period of 1 min and detection at m/z of 195, corresponding to the caffeine molecular ion. The resulting misergram shows the kinetic profile of the appearance of caffeine in the brewed tea. Inclusion of several caffeine standards allows approximate quantitation. A maximum caffeine concentration of about 0.1 mg/mL is reached by about 5 min. It should be noted that the steeping tea samples were not stirred, and that more rapid extraction of caffeine may be possible when teabags are stirred or "dunked."
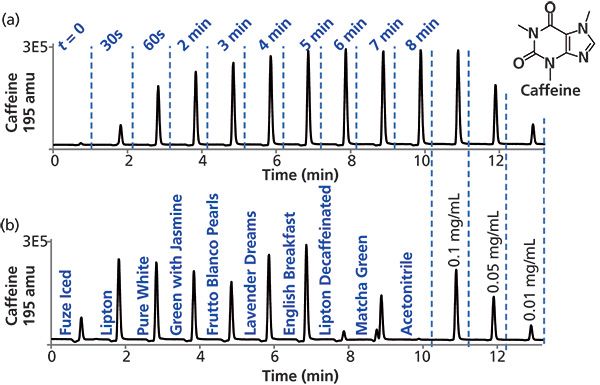
Figure 2: MISER HPLC–MS analysis of caffeine in various teas. Analysis of samples and authentic standards by MISER HPLC–MS with selected ion monitoring of the caffeine molecular ion at 195 amu reveals relative caffeine levels of time-course extraction study from (a) Lipton all natural tea and (b) nine different teas. Chromatographic and MS conditions were as described in the experimental section. Detection: ESI-MS(+) at m/z = 195 amu; injections: 0.6 µL every 1 min.
A comparison of several different commercial teas, each prepared with 5 min steeping is shown in Figure 2b. An iced tea beverage is also included for comparison. While most of the teas show a caffeine level of about 0.1 mg/mL, the level in the iced tea is significantly less, and perhaps not surprisingly, only a trace amount of caffeine is observed in the sample of decaffeinated tea included in the study. In addition, it should be noted that the loose teas studied differed in particle size from large (Frutto Blanco Pearl), to small (English Breakfast tea ground for the Flavia brewing system), to almost powder-like (Matcha green tea). These results are in agreement with previous studies where caffeine concentrations in white, green, and black teas ranged from 14 to 61 mg per serving (6 or 8 oz) (23). Taken together, the MISER LC–MS experiment provides an easily interpreted graphical depiction of the experimental results.
An advantage of the MISER LC–MS analysis is the ability to simultaneously monitor the presence of several different analytes. We next set out to exploit this capability, focusing on the simultaneous evaluation of caffeine, aspartame, and taurine in soft drinks and energy drinks. Accurate quantitation with the MISER LC–MS technique requires analytes of interest to be chromatographically resolved from potentially interfering substances in the sample mixture. When several analytes are simultaneously analyzed, it is important to demonstrate that the short analysis times for MISER LC–MS do not lead to sample interference. Model experiments can help to rapidly assess potential interference issues, identifying any possible "crosstalk" between the principal analytes under investigation. Figure 3a shows the results of such a method development study, clearly illustrating that the shorter method leads to a significant ion suppression of caffeine and taurine MS signal in the presence of aspartame, while the longer 1-min injection period is free from such problems.
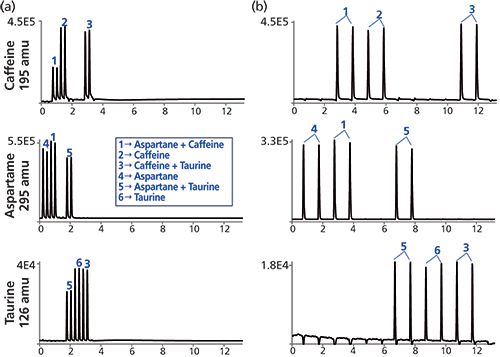
Figure 3: Signal response comparisons ( m/z 195, 295, and 126) for caffeine, aspartame, and taurine standards and the respective two component mixtures: (a) MISER HPLC–MS profiles obtained from a fast method: column: SB-C18 (20 mm × 3.0 mm, 1.8 µm); temperature: 40 °C; detection: SIM ESI-MS(+) at m/z = 195, 295, and 265 amu; sample: 0.6-µL injection of each component solution at 0.25 mg/mL in acetonitrile–water; flow rate: 1 mL/min; isocratic mobile phase: 10% eluent A: 2 mM ammonium formate in water (pH 3.5) and 90% eluent B: 2 mM ammonium formate in 10:90 water–acetonitrile (pH 3.5). Time between injections: 22 s. (b) MISER HPLC–MS profiles obtained from the standard method as describe in the experimental section. Time between injections: 1 min.
The simultaneous evaluation of caffeine and aspartame in various soft drinks is shown in Figure 4. No caffeine signal was observed for the orange soda or Pepsi caffeine-free products, which was consistent with the labels on these products. The levels of caffeine observed in Coke, Diet Coke, and Pepsi were found to be slightly higher than 0.1 mg/mL. Pepsi Max was shown to have about 0.2 mg/mL, in keeping with reported values (24).
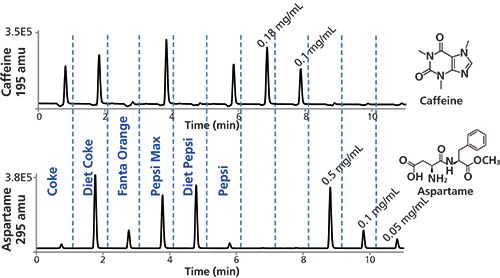
Figure 4: MISER HPLC–MS analysis of caffeine and aspartame in various sodas. Analysis of samples and authentic standards by MISER HPLC–MS with selected ion monitoring of molecular ions at 195 and 295 amu. Experimental conditions as described above. Detection: ESI-MS(+) at m/z = 195 and 295 amu; injections: 0.6 µL every 1 min.
The levels of aspartame in Diet Coke and Diet Pepsi were found to be about 0.5 mg/mL, in keeping with reported values (25). Very small peaks for aspartame (<< 0.05 mg/mL) were observed in both Coke and Pepsi, although neither of these products contain aspartame as a listed ingredient. This finding highlights one of the potential pitfalls of using MISER LC–MS for detecting trace amounts of analytes in very complex mixtures: It may well be the case that the complex caramel mixtures or other components in these colas may contain a component that gives rise to a small amount of a coeluted component with mass 295 amu. Such interference problems could potentially be reduced or eliminated by the use of high-resolution MS detection using exact mass or multiple reaction monitoring (MRM), or the application of selected reaction monitoring (26,27) to multiple product ions from one or more precursor ions (28,29).
We next investigated the analysis of caffeine, aspartame, and taurine in a variety of different "sports energy" drinks, which have risen tremendously in popularity in recent years. In addition to caffeine, energy drinks often contain additives ranging from high-fructose corn syrup (for non-diet versions) to taurine, B vitamins, sucralose, aspartame, maltodextrin, glucuronolactone, carnitine, creatine, inositol, and various extracts of açaí, ginseng, and Ginkgo biloba (30).
The misergrams shown in Figure 5 show the relative levels of caffeine, taurine, and aspartame in a variety of different energy drinks, with Diet Coke and Coke included for comparison. Initial evaluations by direct sample injection showed much higher levels for the three components, with resulting poor baselines. Consequently, all samples were diluted 10 times with water, with the exception of Red Line Xtreme and 5 Hour Energy, which were diluted 100×. The resulting misergrams show much higher caffeine content in the energy drinks than in Coke and Diet Coke, with 5 Hour Energy showing a considerably higher amount of caffeine (similar signal response as Rock Star drinks, but the sample is 10 times more diluted). Red Bull sugar free and Total Zero show aspartame concentrations lower, but comparable to Diet Coke. Rock Star shows almost no aspartame, but checking the ingredients we found sucralose, another artificial sweetener commonly used in energy drink preparations. On the other hand, the taurine signal response from Rock Star, Monster, and Red Bull drinks were very similar at about 0.4 mg/mL, but higher than AMP Boost Cherry. All of these results are consistent with the caffeine, aspartame, and taurine levels published in the different literature sources (24,25,31–35).
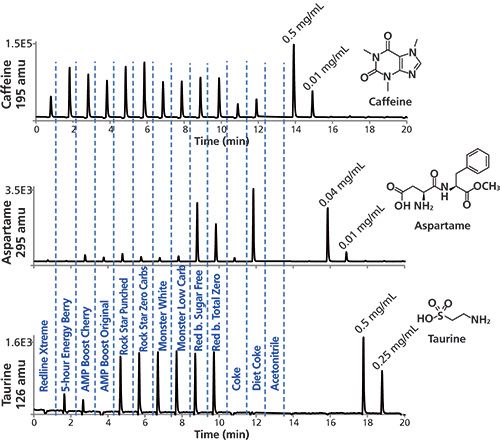
Figure 5: MISER HPLC–MS analysis of caffeine, aspartame, and taurine in various energy drinks and sodas. Analysis of samples and authentic standards with selected ion monitoring of the caffeine, aspartame, and taurine molecular ions at 195, 295, and 126 amu, respectively. Experimental conditions were as described in the text. Detection: ESI-MS(+) at m/z = 195, 295, and 126 amu; injections: 0.6 µL every 1 min. The first two energy drink samples (Extreme Triple Berry and 5 Hour Energy Berry) were 100× diluted, while all of the other samples were 10× diluted.
During the course of our investigations it became clear that in addition to caffeine, aspartame, and taurine, many other components are present in some of these beverages. Many products contain vitamins (such as vitamins C, B5, B6, and B12), artificial sweeteners such as sucralose or even added compounds such as açaí, choline, various forms of ginseng, inositol, carnitine, creatine, and Ginkgo biloba. Figure 6 illustrates how the MISER LC–MS approach can potentially be used for rapid investigation of the presence of some of these additional components, although additional analysis and investigation of potential interference issues would be needed for definitive studies.
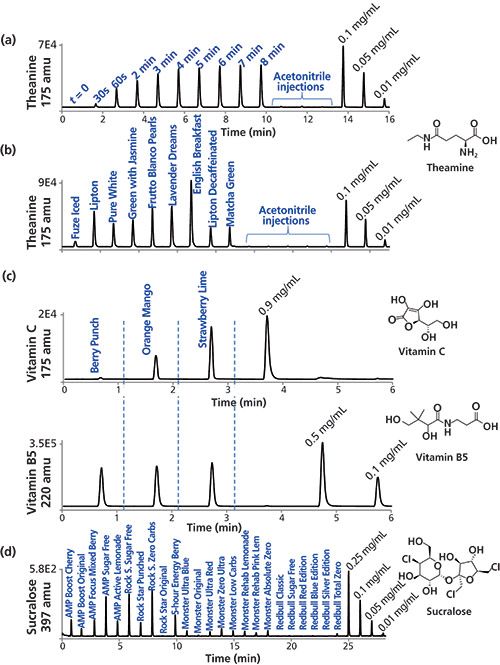
Figure 6: MISER HPLC–MS analysis of multiple analytes in different samples: Theanine levels of time-course extraction study from (a) Lipton all natural tea and (b) nine different teas. (c) Vitamin C and B5 levels of three different vitamin waters. (d) Sucralose levels in 24 different energy drinks (all samples were 10× diluted, except AMP and Rock Star drinks). Chromatographic and MS conditions as described in the experimental section. Detection: ESI-MS(+) at m/z = 175 (theanine) and 220 amu (vitamin B5); ESI-MS(-) at m/z = 175 (vitamin C) and 397 (sucralose) amu, 0.6-µL injection every 1 min. Fragmentor voltage was kept at 150 eV for all compounds, except for vitamin C (100 eV).
Theanine, a nonprotein derived amino acid, is a component of tea that has received considerable attention recently (36–38). The time-course analysis for extraction of this component in English Breakfast tea is shown in Figure 6a, with the level reaching a maximum value at about 5 min, similar to what was observed with caffeine (Figure 2a). The level of theanine in nine different teas is shown in Figure 6b. The level in the bottled iced tea drink is seen to be quite low, a result that is consistent with the report that theanine tends to be highest in freshly brewed teas, with degradation over time being observed in bottled teas.
The levels of vitamins C and B5 in three different vitamin waters are shown in Figure 6c. Similar amounts of vitamin B5 (about 0.2 mg/mL) are seen in all three products, whereas dramatically different amounts of vitamin C are observed. The latter result may be an artifact caused by interference, as reported values suggest that the vitamin C content of Berry Punch should be only about 30% lower than that of Orange Mango. Finally, the evaluation of sucralose in a variety of energy drinks (Figure 6d) shows the highest levels for AMP Sugar Free, Rock Star Sugar Free, and Rock Star Zero Carbs; all of them were reported to contain this artificial sweetener. This is also consistent with the observation that no aspartame was detected in Rock Star drinks (Figure 5).
The advantage of the MISER LC–MS (high throughput, simple readout of results) for simultaneous semiquantitative evaluation of caffeine, taurine, and aspartame levels in teas, soft drinks, and energy drinks can provide an entertaining introduction to the important analytical technique of LC–MS. While this approach is fast and simple, and easily grasped by students, it is important to note that MISER LC–MS is typically used for the analysis of relatively clean samples containing only a few potentially interfering substances, and may be less well suited for reliable quantitation when dealing with samples with highly variable and complex matrices. When more exact quantitation is required, model experiments can help to rapidly assess potential interference issues (39–41).
Conclusions
The approximate levels of caffeine, aspartame, and taurine in soft drinks and energy drinks can be easily measured by MISER LC–MS, providing a useful introduction to the important analytical techniques of liquid chromatography and mass spectrometry. Considerable variation in these analyte levels is clearly visualized among a variety of these beverages, with the energy drinks showing a higher concentration of caffeine and taurine, and the diet soft drinks containing a higher amount of artificial sweetener (aspartame).
Acknowledgments
We are grateful to MRL Postdoctoral Research Fellows Program for financial support provided by a fellowship (E.L.R) and also to the MRL Process and Analytical Chemistry Laboratory for making available the use of the HPLC–MS system used in this study.
References
(1) S.J. Hird, B.P.Y. Lau, R. Schuhmacher, and R. Krska, TrAC, Trends Anal. Chem.59, 59 (2014).
(2) R. Nageswara Rao, Expert Rev. Proteomics 11, 425 (2014).
(3) P. Li and M.G. Bartlett, Anal. Methods6, 6183 (2014).
(4) J. Zheng, J. Mehl, Y. Zhu, B. Xin, and T. Olah, Bioanalysis 6, 859 (2014).
(5) E.L. Regalado et al., J. Chromatogr. A1218, 7358 (2011).
(6) K. Wilczewska, A. Kot-Wasik, and J. Namiesnik, Crit. Rev. Anal. Chem.43, 148 (2013).
(7) E.L. Regalado, R.K. Dermenjian, L.A. Joyce, and C.J. Welch, J. Pharm. Biomed. Anal.92, 1 (2014).
(8) S.-L. Lin, T.-Y. Lin, and M.-R. Fuh, Electrophoresis35, 1275 (2013).
(9) M.R. Gama, C.H. Collins, and C.B.G. Bottoli, J. Chromatogr. Sci.51, 694 (2013).
(10) L.R. Ruhaak et al., Anal. Bioanal. Chem. 405, 4953 (2013).
(11) A. Rocco, A. Maruska, and S. Fanali, J. Sep. Sci.36, 421 (2013).
(12) G. Desmet and S. Eeltink, Anal. Chem.85, 543 (2013).
(13) L. Rieux, E.-J. Sneekes, R. Swart, and M. Swartz, LCGC North Am.29, 926 (2011).
(14) C.J. Welch et al., Tetrahedron: Asymmetry 21, 1674 (2010).
(15) W. Schafer, X. Bu, X. Gong, L.A. Joyce, and C.J. Welch, in Comprehensive Organic Synthesis, C.J. Welch, Ed. (Elsevier, Oxford, UK, 2014) 9, pp. 28–53.
(16) A. Bellomo et al., Angew. Chem., Int. Ed. 51, 6912 (2012).
(17) R. Papp, U. Andersson, and L.-D. Cantin, J. Pharm. Biomed. Anal.77, 94 (2013).
(18) J.P. Vistuba et al., J. Chromatogr. A1274, 159 (2013).
(19) C.J. Welch, E.L. Regalado, E.C. Welch, I.M.K. Eckert, and C. Kraml, Anal. Methods6, 857 (2014).
(20) J.E. DiNunzio, J. Chem. Educ.62, 446 (2014/08/06, 1985).
(21) R.E. Leacock, J.J. Stankus, and J.M. Davis, J. Chem. Educ.88, 232 (2014/08/06, 2011).
(22) E. Bier Mark and J. Grabowski Joseph, in Active Learning (American Chemical Society, 2007) 970, pp. 171–187.
(23) J.M. Chin, M.L. Merves, B.A. Goldberger, A. Sampson-Cone, and E.J. Cone, J. Anal. Toxicol.32, 702 (2008).
(24) Caffeine Informer. Caffeine Content of Drinks. http://www.caffeineinformer.com/the-caffeine-database. Retrieved August 2, 2014.
(25) N.R. Ekere, Int. J. Appl. Chem. 8, 129 (2012).
(26) E. de Hoffmann, J. Mass Spectrom. 31, 129 (1996).
(27) P. Picotti and R. Aebersold, Nat. Meth.9, 555 (2012).
(28) R.W. Kondrat, G.A. McClusky, and R.G. Cooks, Anal. Chem.50, 2017 (1978).
(29) R.D. Unwin et al., Mol. Cell. Proteomics 4, 1134 (2005).
(30) B. Meier (January 1, 2013), "Energy Drinks Promise Edge, but Experts Say Proof Is Scant." The New York Times. Retrieved January 2, 2013.
(31) J.P. Higgins, T.D. Tuttle, and C.L. Higgins, Mayo Clin. Proc. 85, 1033 (2010).
(32) M. Hohmann et al., J. Pharm. Biomed. Anal.93, 156 (2014).
(33) H.K. Khurana, I.K. Cho, J.Y. Shim, Q.X. Li, and S. Jun, J. Agric. Food Chem.56, 778 (2008).
(34) L.C. Tamamoto, S.J. Schmidt, and S.-Y. Lee, Journal of Food Science75, S271 (2010).
(35) M. Aranda and G. Morlock, J. Chromatogr. A1131, 253 (2006).
(36) M.J. Desai and D.W. Armstrong, Rapid Commun. Mass Spectrom. 18, 251 (2004).
(37) R. Cooper, Int. J. Food Sci. Nutr.63, 90 (2012).
(38) Q.V. Vuong, M.C. Bowyer, and P.D. Roach, J. Sci. Food Agric. 91, 1931 (2011).
(39) B.K. Matuszewski, M.L. Constanzer, and C.M. Chavez-Eng, Anal. Chem.75, 3019 (2003).
(40) S. Murugan, N. Pravallika, P. Sirisha, and K. Chandrakala, J. Chem. Pharm. Sci.6, 41 (2013).
(41) A. Furey, M. Moriarty, V. Bane, B. Kinsella, and M. Lehane, Talanta115, 104 (2013).
Christopher J. Welch, Erik L. Regalado, and Michael H. Kress work in process and analytical chemistry at the Merck Research Laboratories in Rahway, New Jersey. Christina Kraml, E. Celeste Welch, Margaret J. Welch, Hannah Semmelhack, Danielle Almstead, Abigail S. Kress, and Natacha A. Hidalgo are with Lotus Separations with the Department of Chemistry at Princeton University in Princeton, New Jersey. Direct correspondence to: christopher_welch@merck.com and erik.regalado@merck.com

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.
A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.












