Meeting Review: Advances in Bio-Separations: Biologics Characterization from Cradle to Grave (Day One)
Paul Ferguson1 and W. John Lough2, 1The Chromatographic Society, Glasgow, UK, 2University of Sunderland, Sunderland, UK.
Photo Credit: Rafe Swan/Getty Images

A two-day symposium, organised by The Chromatographic Society, focusing on the characterization of biopharmaceutical molecules at AstraZeneca’s MedImmune site in Cambridge, UK, brought together renowned external speakers and delegates from academia, pharmaceuticals, and scientific instrument/technology companies alongside MedImmune colleagues. The programme covered the analysis of peptides, oligonucleotides, and monoclonal antibodies (mAbs), proposing multiple approaches towards the successful analysis of these types of molecules.
The Chromatographic Society Spring Symposium and Annual General Meeting was held at MedImmune’s Granta Park site on Tuesday 12 and Wednesday 13 of May. This meeting on biopharmaceuticals was something of a landmark. The separation of biopharmaceuticals had been addressed in previous meetings, but there had often been blurring at the edges with bio-separations also taken to encompass small molecule drug bioanalysis. This was the Society’s first (two-day) meeting devoted exclusively and unambiguously to the separation of large biological molecules of pharmaceutical interest. The interest in this increasingly important area for the pharmaceutical industry was reflected in the attendance of over 120 delegates across different companies in attendance. In addition, around 35 vendors representing 16 companies, including the principal meeting sponsors Shimadzu, presented the latest from their product portfolios making for a vibrant atmosphere.
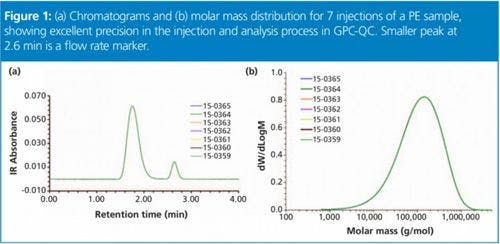
Tony Taylor (Honorary Secretary, The Chromatographic Society) opened the meeting providing a brief outline of the Society and its activities and set the context for the meeting. He noted that the boundaries between small and large molecule analysis are becoming more blurred as small molecule chromatographers increasingly need to perform large molecule analysis (for example, for short chain peptides and oligonucleotides) and vice versa (unbound “payload” on antibody-drug conjugates [ADCs]), and that this meeting would attempt to address that overlap. The increasing importance of large molecule pharmaceuticals is highlighted in Table 1 showing that seven of the top 10 selling drugs in 2014 were biomolecules.
The audience was a mix of biopharmaceutical separations specialists and those more familiar with small drug molecule separations but anxious to learn about the burgeoning large bio-molecule area - the former in particular anxious to get down right away to the nitty gritty of separations. However, this was a well-structured meeting and it was important to go beyond Tony’s introduction to underscore the strategic significance of biopharmaceuticals for the future of the pharmaceutical industry. Accordingly, in the opening session (also featuring Tony as Chair), Dr Alistair Kippen (Director - Analytical Biotechnology in Biopharmaceutical Development, MedImmune) gave an insight into the history of MedImmune in his talk “Introduction to MedImmune Cambridge and Analytical Biotechnology” (Launched in 1991; steady evolutionary growth with a series of acquisitions followed by acquisition of the company itself by AstraZeneca [AZ] in 2007), their historical product-line achievements (an impressive track record of “firsts” in biopharmaceutical development such as AbbVie’s Humira, the first fully human antibody to be marketed, alongside significant collaborations with pharmaceutical giants such as Merck and GSK), and an overview of the development process of monoclonal antibody therapeutics (mAbs). He provided an oversight of the process, which included the production of a cell line, manufacturing, and scale-up (“upstream processing”), purification (“downstream processing”), and also formulation and analytical activities to support the process. He stated that the analytics involved with the development of novel biologics are aligned with the key structure-functional attributes and stability of the molecule. The critical quality attributes (CQAs) tested include size (the measurement and control of aggregation is a key issue), charge heterogeneity, primary sequence, function (potency), higher order structure, and post translation modifications. He then went on to highlight the variety of new products being developed based on mAb technology including protein fusions and ADCs, all of which present unique analytical challenges. The importance of biotherapeutics to AstraZeneca’s growth was highlighted in his comment that approximately 50% of the company’s development portfolio is large molecules.
This theme was extended beyond the AZ/MedImmune context by the next speaker, Professor Gary Walsh of the University of Limerick, Ireland, and a recognized authority in the field of biopharmaceutical characterization including regulatory aspects. His talk was titled “Biosimilar characterization for regulatory approval” and continued to set the scene on the importance of this class of molecules to the pharmaceutical industry. For many years there have been predictions of a global top 10 drugs by sales dominated by biopharmaceuticals and that has come to fruition. He noted that since synthetic insulin was first brought to the market in 1982 (marketed as Lantus by Sanofi), 255 biopharmaceutical molecules (principally proteins) have been approved for human use and an estimated 2000 products are in development globally. Prof. Walsh then went on to define the regulatory implications for developing and filing biosimilar compounds (essentially molecules that are structurally and clinically similar to market approved mAbs) and the analytical characterization required. For a biosimilar marketing authorisation application (MAA), a full quality module including reduced clinical and non-clinical datasets is required alongside comparative data to the originator product where structural differences between molecules have been identified, the implications considered, and the significance assessed. He concluded by focusing on biosimilar mAbs and outlining the important critical quality attributes (CQAs), which include primary and higher order structure, glycosylation, oxidation/deamidation/terminal lysine differences, aggregation, HCP (host cell protein) analysis and bioactivity, and the analytical techniques (primarily chromatographic) used to determine any differences. He remarked that development of biosimilars typically leads to cost savings of 20–30% over the originator product and is often important to driving innovation in the originator companies.
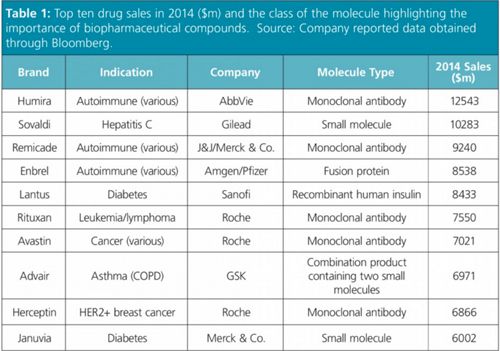
Figure 1: Levels at which protein based biopharmaceuticals may be characterized (reproduced with permission).
After lunch and the first exhibition session opportunity to interact with the vendors present at the meeting, Dr Koen Sandra from the Research Institute for Chromatography, Belgium, opened the afternoon’s proceedings with a very detailed but clear presentation on “Strategies for the characterization of biopharmaceuticals: protein, peptide, and glycan analyses”. He began with a short overview on mAb biopharmaceuticals including typical characteristics of these molecules, the “levels” at which they can be characterized (Figure 1) and the techniques commonly employed to analyze these. At the protein level, reversed-phase liquid chromatography–mass spectrometry (LC–MS) can be used to analyze the intact protein, light and heavy chains, and the Fab (fragment antibody)/Fc (fragment crystallizable) typically using shallow 0.1% TFA/acetonitrile mobile phases gradients on long, small particle columns at low flow rate and high temperatures (~80 ºC). This was exemplified with an analysis of Remicade (infliximab, a monoclonal antibody for the treatment of autoimmune diseases, first approved by the FDA in 1998 for use against Crohn’s Disease) and its biosimilar.
Dr Sandra then went on to discuss the analysis at the protein level using ion-exchange chromatography, which is typically used to analyze charge variants of the protein, and size-exclusion chromatography (SEC), which is used to identify potential aggregates (dimeric and higher order structures). He followed this logically by then focusing on analysis at the peptide level following trypsin enzymatic digestion. This is required because analysis at the protein level does not provide the complete structural picture, nor does it allow understanding of localized modifications such as which amino acid is glycosylated, oxidized, deamidated. He exemplified this through analysis of digested Herceptin (trade name for the breast cancer drug, trastuzumab) using similar conditions to those at the protein level illustrating ~99% coverage of all the peptides in the protein and highlighting glycosylation differences with a biosimilar. MS–MS is critical to this analytical approach helping to pinpoint residue changes and identify mutation sites. Dr Sandra also highlighted the value of two-dimensional (2D)-reversed-phase LC for comparability assessment at the peptide level.
Again logically, the focus of the final part of his talk was on analysis at the glycan level. Here the hydrophilic interaction liquid chromatography (HILIC) mode of LC is commonly employed for the polar analytes involved. The glycans are liberated from the Fc region of the mAb using PNGase F and then tagged with 2-aminobenzamide for sensitive fluorescence and MS detection. Long, small particle amide columns with ammonium formate/acetonitrile mobile phases are typically employed for this type of analysis. He completed his presentation by showing how a biosimilar glycosylation could be brought within originator specifications by adding uridine, galactose, and manganese to the CHO (cell line) growth medium, which would otherwise not have been approved.
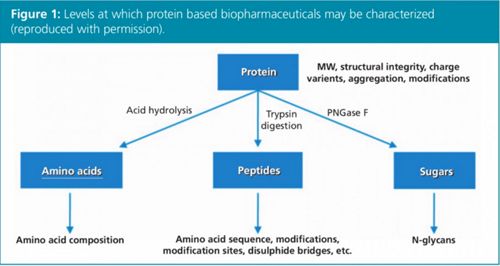
Figure 2: Impact of temperature and increased pressure on mAb aggregate formation (reproduced with permission).
The subsequent speaker, Dr Davy Guillarme from the University of Geneva, Switzerland, covered similar ground but approached it more from a mode of LC standpoint, eloquently discussing “Reversed-phase, size-exclusion, and ion-exchange chromatography for biomolecules characterization”. The first mode he addressed was SEC, which was used for the determination of protein/mAb aggregates. He discussed the trend in moving towards higher temperatures and smaller particle size columns leading to improved mass transfer and chromatographic performance. However, he stated that care must be taken not to induce aggregation under high temperature or higher pressure conditions (Figure 2).
Dr Guillarme then went on to discuss ion-exchange chromatography (IEX), with an emphasis on cation exchange, which dominates because mAbs generally possess a high pI (isoelectric point). Cation exchangers with salt gradients are commonly employed for the analysis of mAb charge variants and he proposed a generic method of a 100 × 4.6 mm column and a salt gradient using 10 mM MES pH 5.7 and 10 mM MES pH 5.7 containing 1 M NaCl, or a 100 × 4.6 mm column and a pH gradient mobile phase of a buffer at pH 5.6 and a buffer pH 10.2 for intact mAb characterization. These essentially generated the same results but the salt gradient was easier to perform.
He then went on to discuss the application of reversed-phase LC for the analysis of biopharmaceuticals. He noted the issues involved in using this approach, namely peak tailing, peak broadening, and adsorption, and offered solutions to this, for example, use of ion-pairing reagents, use of wide pore particle columns, and use of less hydrophobic stationary phases. The final part of his presentation focused on the analysis of mAbs and complemented the data presented by Dr Sandra in the previous lecture, reiterating the need for high temperatures and wide pore materials with C4 or C8 alkyl bonding for successful analysis of these molecules. However, care should be taken as long gradients at high temperatures can be detrimental to mAb stability and occasionally thermal degradation is observed. In his final slide he also discussed the impact of high pressures (achieved by increasing flow rate) and temperatures on a selection of mAbs, illustrating that the impact can be non-linear as the protein unfolds and denatures.
The next talk involved a change of tack towards MS. Dr Alexander Makarov from Thermo Scientific, Germany, (ex-Warwick University and inventor of the high resolution OrbiTrap MS) delivered a very entertaining and informative presentation on the development of the OrbiTrap and its application to biopharmaceutical characterization (“Bio-Chromatography with Orbitrap detection: past, present and future”). After discussing the development and challenges in developing such a high-resolution instrument, he described how this technology has expanded into a range of Orbitrap products. He also described experiments undertaken within his laboratory where mass resolution values of >1,000,000 have been achieved! Importantly, Dr Makarov then went on to describe the applications of this technology with examples in peptide mapping (including an impressive 100% sequence coverage from a 5 min LC–MS analysis of digested Rituximab, mainly a rheumatoid arthritis treatment), determination of drug/intact mAb ratios for ADCs, and measurement of antibody-antigen binding. He completed his presentation with a discussion of future possibilities for the technique by presenting data on the analysis of intact virus particles as drug delivery systems.
Dr Viv Lindo (MedImmune) also highlighted the value of MS detection during his presentation on “Applying the product characterization toolbox: quality attribute-based testing for non-mAbs”. The focus of this was on four discreet areas: (i) quality attribute based testing; (ii) intact mass challenges for non-mAbs (iii) size and instability challenges for non-mAbs; and (iv) glycosylation challenges. He proceeded by discussing the vast array of analytical techniques that could (and are often) applied to the analysis of mAbs, but proposed that the testing strategy should be governed by the quality attribute measurement required. He then discussed how MS detection is critical to mAb biopharmaceutical characterization, but pointed out that the correct approach should be carefully selected. While fragment-based analysis of a mAb using a quadrupole time-of-flight (QTOF) mass spectrometer was possible, using the same approach for a recombinant glycoprotein may yield much poorer results. He suggested that for extremely heterogeneous systems matrix-assisted laser desorption ionization (MALDI-) TOF with in-source decay was a more appropriate approach for sequence mapping. Dr Lindo then went on to discuss size and instability issues associated with a recombinant protein that was three polypeptide dimers held together by non-covalent association. This protein dissociates readily in MS-compatible solvents but was successfully analyzed by MALDI-TOF after primary amine cross-linking of monomers (CovalX technology), which does not form aggregates. The final part of his talk focused on glycosylation challenges with heterogeneity. He demonstrated the successful use of 2-aminobenzamide labelling (with HILIC chromatographic methodology) for base permethylation for the analysis of glycoproteins.
The invited speaker programme was interspersed with presentations from a a variety of vendor companies. Their presentations focused on a vast array of approaches and considerations for characterizing these molecules. The vendors were also present in the exhibition space where representatives were on hand to discuss their latest product ranges and offer technical advice.
Figure 3: Speakers Dr Davy Guillarme (left) and Dr Koen Sandra (centre) with Chromatographic Society Committee member, Dr James Heaton at the Imperial War Museum Duxford

Figure 4: Organizers, delegates, and vendors socialising at the symposium dinner held at the Imperial War Museum Duxford

The symposium dinner was held at the Imperial War Museum Duxford - the organization’s largest aeronautical museum. The dinner was sponsored by Shimadzu, Crawford Scientific, and VRS and included a tour of the main hanger followed by dinner. Dr Paul Ferguson (President, The Chromatographic Society) gave a short after-dinner speech thanking the meeting sponsors and reflecting on the heritage, current focus, and future direction of the Society. This was followed by an aviation quiz, which was won by Stuart Phillips of Shimadzu. While a good time was had by all, it has to be noted that such events are not just a frivolous sideshow but are integral to every meeting - playing a vital role in facilitating networking. The Duxford dinner was very much a success in this respect as well as being something a bit different.
The programme for Day Two of this illuminating conference will be covered in the next issue of The Column.
E-mail: chromsoc@meetingmakers.co.uk
Website: www.chromsoc.com

New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)