Changing from Helium and Nitrogen While Maintaining Separation Efficiency and Analysis Time
Wouldn’t it be nice if we could make nitrogen work for applications where helium is not an option?
Most scientists in the world of gas chromatography (GC) will advise you not to use nitrogen because it is considered a “dinosaur” gas, and instead use helium, which offers shorter analysis time. But, wouldn’t it be very nice if we could make nitrogen work for applications where helium is not an option?

Hydrogen is a popular choice of carrier gas in many gas chromatography (GC) methods because it gives the shortest analysis time while nitrogen gives the longest (see Figure 1). However, one always runs into the discussion of safety when using hydrogen and this is an on-going challenge for many laboratories that choose not to use hydrogen because of liability. Scientists therefore need to be aware of what the impact will be on a separation when changing the carrier gas named in a method.
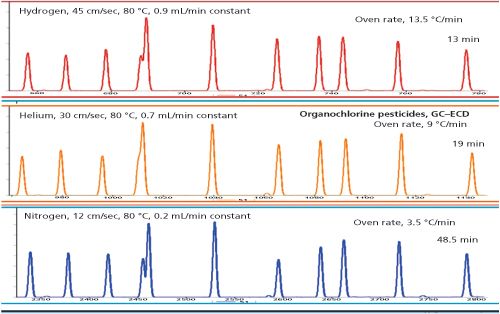
Figure 1: Example of samples analyzed using three carrier gases under optimal conditions. The chromatograms are similar because the elution temperatures are the same. Nitrogen analysis takes a long time because of low optimal velocity, but gives more plates as can be seen on the separation/elution of the 4th set of peaks.
Figure 1 shows that when operated under optimal conditions the three main carrier gases - nitrogen, hydrogen, and helium - result in the same separations and the biggest impact is analysis time. The challenge when switching between carrier gases is “How can I maintain efficiency using (for example) nitrogen as carrier gas operating at a higher velocity?” The answer? Make sure that you compensate for the loss of plates by using a smaller diameter capillary column. For example, if you replace a 30 m × 0.25 mm capillary column for a 20 m × 0.15 mm version that has the same phase ratio, the same amount of plates can be generated in the same amount of time. The other interesting point is that this can be realized using the same oven programming conditions.
The unique features that this concept can offer are:
• Separations are exactly identical.
• Analysis time is the same.
• No change in oven temperature programme.
• The pressure required for nitrogen is just a little higher.
• Nitrogen is always available and can even be produced in the laboratory.
• Price advantage for nitrogen and volume used.
• 0.15-mm columns have the same outside diameter (OD) as 0.25 mm, which means you can use the same ferrules.
• 0.15 mm columns have been proven to work for many years.
This article shows that the concept works for both polar and non-polar stationary phases. We also show that despite the reduced capacity, 0.15-mm i.d. columns can be used for tough sample matrices and still obtain decent life times.
How to Select the Oven Temperature Programme
Selection of the optimal oven temperature programme is very important when changing carrier gas because the linear velocity of the carrier gas is changed and components are carried through the column at a different temperature. The impact of different elution temperatures can be great, with peaks appearing in different positions of the chromatogram that can even swap around. This is a physical process and will happen with all stationary phases.
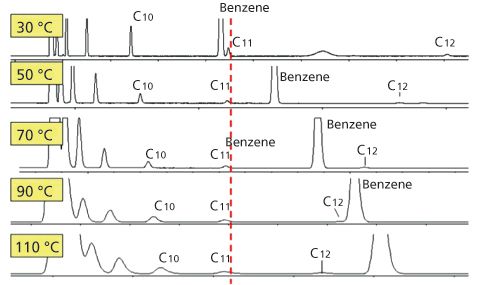
Figure 2: Hydrocarbon and benzene mixture analyzed on a capillary column at different temperatures. Note that the polarity of the column increases with temperature.
Figure 2 shows an example of where a mixture was analyzed using different isothermal temperature conditions using a very polar TCEP-capillary column. The resulting chromatograms are totally different. At 40 °C the benzene elutes before the C11, while at 110 °C, the benzene elutes after C12. If this sample is analyzed at 140 °C the benzene will elute after C13. This shows that separation selectivity is directly related to elution temperature, which is to be expected because interactions with the stationary phase at higher temperatures will be different to interactions at lower temperatures.
When changing column dimensions or carrier gas type, it is preferable to obtain the same chromatogram as in the original method. However, this is only possible if the elution temperature of the components remains the same. Isothermal methods are simple, but the majority of methods are temperature programmed and so the whole temperature profile of the column needs to be translated. As carrier gases can be compressed and different pressures are required, this calculation can be quite complex. Blumberg recognized this and a software program was made available.1 Recently, a new (free) translator has become available that can be accessed from the web and also can be downloaded as a windows program.2 Such calculations are essential if the user wants
• To optimize their present conditions of separation.
• A faster analysis (change of flow).
• To change the column dimensions (length, diameter).
• To move from a flame ionization detection (FID) to a mass spectrometry (MS) method (vacuum will add velocity).
• To work with another carrier gas (nitrogen or hydrogen).
• A combination of the above.
Using Nitrogen at a Higher Linear Velocity (Speed of Helium)
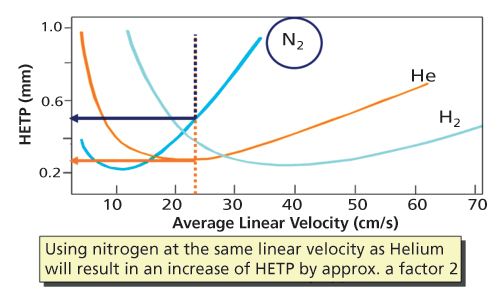
Figure 3: Van Deemter curves showing HETP loss when nitrogen is used under the optimal velocity of helium.
If nitrogen is operated under optimal flow conditions, analysis time will be 2–2.5 times longer, which is less than ideal. Nitrogen can also be used at the same velocity as helium. Figure 3 shows what can be expected using the van Deemter curve. There is a loss of theoretical plates of about a factor of 2 because of the higher speed. You can use the same oven conditions as used for helium. The chromatogram will be the same, but the peaks will be a little broader and about 30% lower in intensity. For separations that are “good enough”, this can be an interesting approach. The practical implication one has to realize are:
• Because peaks are broader, the peaks will merge faster upon column ageing. This means that the number of analyses that can be done under these conditions will be smaller. You will have to use more columns and the cost for each analysis will increase.
• For the same reason the maintenance intervals will be shorter.
• Peaks are lower in intensity, so there is a sensitivity loss.
It is also possible to use nitrogen and compensate for the loss of efficiency using a different column diameter.
It has been known for a long time that analysis time can be shortened using a smaller diameter capillary.3 The same separation can be obtained using a shorter length column.
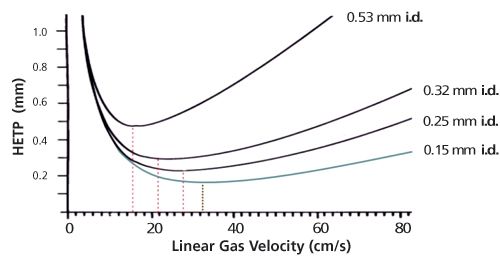
Figure 4: Van Deemter plots for helium and different diameter capillary columns. Optimum velocities move to the higher values with decreasing internal diameter.
In practical terms, 0.15-mm columns have been proven to be very effective and can be coated with different phases, with phase ratios to match the capillaries available in 0.25 mm and 0.32 mm. What makes this exercise especially interesting is that the optimum flow/velocity also increases when internal diameters are reduced (see Figure 4). Columns of 0.15 mm can be operated at relatively higher linear velocity; in addition, the slope of the van Deemter curve is even lower, meaning using N2 at a higher velocity will not cause an extreme loss of plates.
Case Study: Translation from Helium to Nitrogen Using 0.15-mm i.d. Columns

Figure 5: Screenshot of method translation showing the impact on parameters when changing column dimensions and carrier gas from hydrogen to nitrogen. Operating nitrogen at 0.36 mL/min shows the same analysis time using the same programming conditions. 1) Shows the custom option; 2) Shows the carrier gas selection, 3) Column dimensions, original 0.25 m and the new 0.15 mm, 20.2 m; 4) Hold-up times are set at a similar value (gas velocity for nitrogen will be higher then optimal); 5) To check the analysis time, these values should be similar, but should have the exact same run time; 6) Shows the temperature program, which will also be exactly the same.
In this work we used a combination of different carrier gases, a smaller diameter column, and operation at a higher linear velocity. Figure 5 shows a snapshot of the data obtained using EZGC method translation. This is a custom calculation because we wanted to operate the 0.15âmm i.d. column at a higher linear velocity and the method translation was performed in a custom mode.
When the hold-up times are matched, the exact same temperature programme can be used, which results in the same analysis time. Note that the pressures required for nitrogen (17.42 psi) are just a little higher compared with the pressure required for helium (15.27 psi).
The big question is “How much resolution is sacrificed when this exercise is done?” Running nitrogen at a higher velocity will cost efficiency. And what is the benefit of using the SAME temperature programme?
The exercise above was tested with a 30 m × 0.25 mm, 0.25-µm Stabilwax polar fused silica column and a 20 m × 0.15 mm, 0.15-µm Rtx Cl-Pesticides non-polar fused silica column (Restek Corporation). Flowârates and pressures were calculated using the EZGC method translator (Restek).2 The program assists with the conversion of GC settings and offers several choices on optimization.
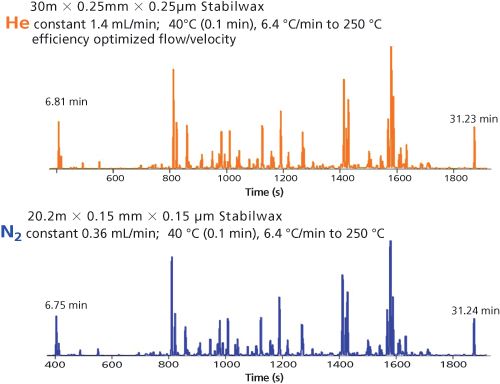
Figure 6: Separation of a fragrance mixture. Separation, void, and analysis time are identical.
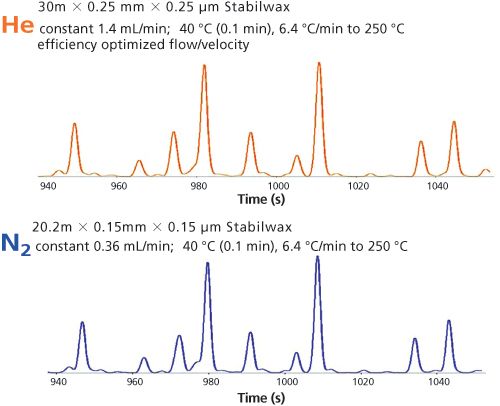
Figure 7: Detail of separation shown in Figure 6, same separation.
The first experiments using the polar fused silica column showed very interesting results. Figure 6 presents the results that are obtained using helium on the 30 m × 0.25 mm, 0.25-µm column and nitrogen on the 20 m × 0.15 mm, 0.15-µm column, using exactly the same programming conditions. The chromatograms seem to match exactly, meaning the separation efficiency of both columns is similar. The analysis times are also exactly the same. Even when we expand the scale, the separations match up very nicely with the polar-wax phase. If this works with polar phases, this concept can also be expected to work with less polar phases.
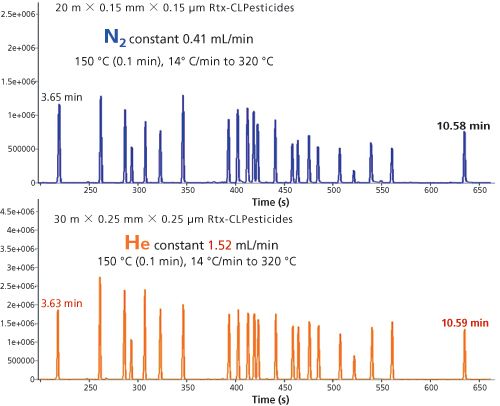
Figure 8: Separation of pesticide mixture. Both chromatograms recorded with exact similar oven temperature program. Separation, void, and analysis time are exactly identical.
The same comparison was performed using a less polar selective phase optimized for separation of chlorinated pesticides. Figure 8 shows the analysis on the 30 m × 0.25-mm column under helium and also on the 20 m × 0.15 mm column using nitrogen. Both columns had the same phase ratio. Even with the less polar phase the separations and analysis times match up very well.
Case Study: Robustness
Columns with a smaller diameter are more sensitive to matrix effects, but in reality it is not so bad.4 If one can use split injection and reduce the absolute amount, smaller bore columns perform very well. When samples are introduced with a stronger matrix (extracts of soil, food, or biological samples), the impact will be bigger.
To test the practical application, we tested a 0.15-mm i.d. column using EPA 8081B with repeated injections of used motor oil. Between the oil injections, a pesticide standard was analyzed. In this analysis, the degree of contamination is measured by the response of degradation product of either DDT or endrin using electron capture detection (ECD). If it exceeds 15%, maintenance is required (liner, cut section of inlet of column).
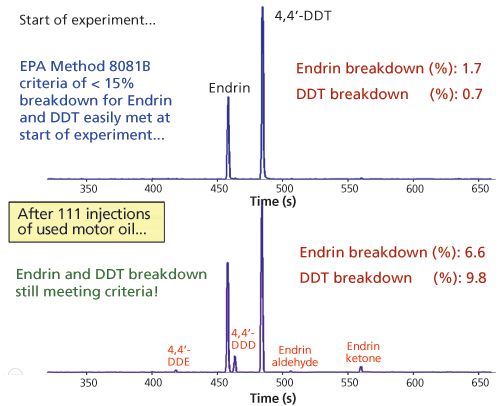
Figure 9: Robustness experiment.
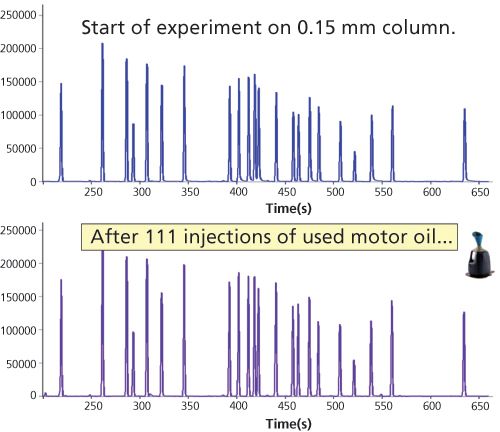
Figure 10: Analysis of pesticide standard before and after the 111 injections of 1 µL of used motor oil.
Figure 9 shows the breakdown result of endrin/DDT after 111 injections of 1 µL of used motor oil; the breakdown still meets the criteria. Note that besides the column, there is also a liner effect included. Figure 10 shows the response of the pesticides standard before and after the experiment.
The response of pesticides as well as the degradation effects because of the motor oil injections is very acceptable, showing that 0.15- mm i.d. columns can be used for tough matrices.
Summary
In summary we have shown that it is possible to replace helium with nitrogen as the carrier gas and obtain very similar separations and analysis times using the same equipment. The only change is to replace the current 0.25-mm capillary column for a 0.15 mm one. Oven conditions can be kept the same and as a result the chromatogram will be similar. Pressure for nitrogen is a little higher compared with helium so the injection conditions are near identical. Installation of 0.15-mm columns is similar to 0.25 mm as the columns have a similar outside diameter of 0.38 mm. The same fittings/ferrules can be used.
It was also shown that 0.15-mm columns show high robustness and can even be routinely used for high matrix samples like used motor oil.
Replacing the 0.25-mm column for a 0.15-mm version and operating under nitrogen will also result in a huge saving on carrier gas, not just for the price of carrier gas, but a 0.15-mm column requires 4× less carrier gas to produce the same results.
References
1. L.M. Blumberg and M.S. Klee, Analytical Chemistry 70(18), 3828–3839 (1998).
2. EZGC Method Translator, http://www.restek.com/ezgc-mtfc [Last accessed: 1 September 2015].
3. J. de Zeeuw and M. Barnes, Am. Lab. December 2005.
4. E. Matisova et al., Journal of Chromatography A1084, 63–70 (2005).
Jaap de Zeeuw
has 36 years of experience in GC capillary technology and has produced more than 100 publications on the topic of GC column technology and applications. He joined Restek (The Netherlands) over nine years ago as an International Specialist on gas chromatography and is currently involved with product development, application, teaching, and marketing.
Jack Cochran
is a Director of New Business and Technology at Restek Corporation. He is a recognized expert in GC and GC×GC for the analysis of pesticides and priority pollutants. He serves on the Board of Directors for FLAG Works (sponsor of NACRW) and the Centre of Oil and Gas Research and Development at the University of Manitoba. Jack is also an Adjunct Professor in the Forensic Science Program at The Pennsylvania State University.

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)