Gel Permeation Chromatography (GPC) for Process Control and Quality Control in Polyethylene and Polypropylene Manufacturing Plants
The Column
A method specifically developed to measure the molar mass distribution (MMD) of polyolefins in a manufacturing plant laboratory has been developed. It is demonstrated that the total cycle time, including the sample dissolution step, can be reduced to around 30 min, well in line with the process control requirements.
A method specifically developed to measure the molar mass distribution (MMD) of polyolefins in a manufacturing plant laboratory has been developed. It is demonstrated that the total cycle time, including the sample dissolution step, can be reduced to around 30 min, well in lne with the process control requirements.
Photo Credit: ©Santiago Urquijo/Getty Images

The family of polyolefins includes high-density and low-density polyethylene (HDPE, LDPE), polypropylene (PP), ethylene-propylene (EP) rubber, and linear low-density polyethylene (LLDPE) copolymers of ethylene with alpha olefins (propylene, 1-butene, 1-hexene, 1-octene). They are polydisperse materials, meaning that a single product is made up of a range of chains with different chain-lengths and possibly different chemical compositions when more than one monomer type is used in the synthesis. Moreover, different molecular architectures (linear or branched) and stereo-structures (tacticity in PP) can occur in these products. All that complexity, together with the semi-crystalline nature of most polyolefin materials, helps to understand the expanding application range of these products.
Different manufacturers around the world use a range of reactor configurations and catalyst systems to produce thousands of tons of polyethylene and polypropylene every year in huge petrochemical complexes. Production is typically controlled on the basis of bulk properties, even if the material produced is characterized by a distribution of molar masses (MMD) and possibly a distribution of chemical compositions (CCD). Melt flow index is perhaps the most important of such bulk parameters because it is closely related to the average molar mass. Density on the other hand is more closely related to the average of chemical compositions and is also used for controlling the reactor and the product.
The classical approach of relying on bulk physical properties for control of the manufacturing process is approaching its limits, with the development of more complex polyolefins (such as multimodal PE or heterophasic PP) and the requirement for a more uniform production and tighter specification ranges demanded by a global and competitive market.
Gel permeation chromatography (GPC) is extensively used to determine MMD in industry analytical laboratories, but the inherent complexity of MMD analysis has restricted it from becoming routine. This is because of the need for high-temperature operation (given the semi-crystalline nature of polyolefin), together with the fragility of columns. In this article, GPC is used to obtain MMD data for process control.
Method
Precision in the determination of molar mass is key to the application of GPC in manufacturing control, so some experiments were conducted to assess whether the performance was satisfactory. A series of replicate analyses of an LLDPE material (density 0.868 g/cm3) were performed in a GPC–QC instrument (Polymer Char) with an infrared detector, using typical analysis conditions. Mobile phase: 1,2,4-trichlorobenzene (TCB) with 300 ppm of butylhydroxytoluene (BHT) as antioxidant; flow-rate: 2.0 mL/min: column and detectors temperature: 150 ºC; injection: 200 μL: column: 150 × 7.5 mm, PL Rapide H (Agilent). For sample preparation, 40 mg of polymer was placed in the vial and the filling volume was set to 80 mL taken from the same reservoir as the mobile phase. Dissolution time was 60 min at 160 ºC with 400 rpm stirring.
Results
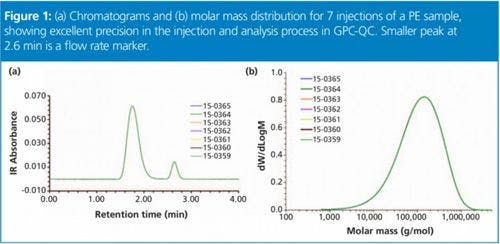
Figure 1: (a) Chromatograms and (b) molar mass distribution for 7 injections of a PE sample, showing excellent precision in the injection and analysis process in GPC-QC. Smaller peak at 2.6 min is a flow rate marker.
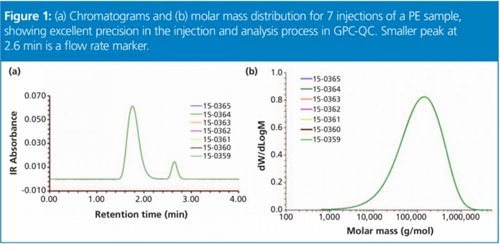
The IR detector concentration chromatograms are shown in Figure 1(a) for the seven replicates that overlay perfectly. It should also be noted that the separation was achieved in a time slightly over 2 min. The total analysis time can be reduced to around 3 min and solvent consumption to around 50 mL for each sample.
Table 1: Summary of molar mass averages and polydispersity index showing excellent precision obtained by GPC.
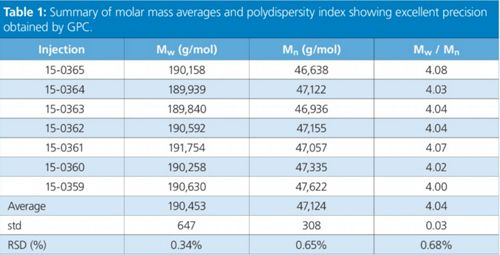
The precision in the collected chromatograms is translated to the calculated MMD as seen Figure 1(b). The molar mass averages Mw and Mn from all individual distributions are presented in Table 1, together with the polydispersity index calculated as the ratio Mw/Mn. This index is related to the broadness of the distribution, and that information is not available when only bulk properties are measured. The precision in the molar mass averages and polydispersity index expressed as standard deviation (std) and relative standard deviation (RSD %) is remarkable for GPC analysis.
The separation was performed in less than 3 min; however, the polymer had to be brought in solution before analysis, which was ensured by using good heat transfer and vigorous stirring. The vial was also filled with preheated solvent, so no additional time was required to increase its temperature once in the vial.
The optimized dissolution and separation method opens the door to high temperature GPC analysis within 30 min in most cases - including the sample preparation step - and less than 1 h even for the most difficult products. This enables practical application in manufacturing plants as a process control or quality control tool.
The use of an IR detector in GPC can also generate useful information on the chemical composition of the sample being analyzed. This is particularly relevant in the case of copolymers that account for a large proportion of polyolefins produced, such as LLDPE, some HDPE, and also the important group of EP copolymers. The information generated by the IR detector can be translated to either co-monomer weight fraction or to density units for a given product range. A single value representing the bulk composition/density can be reported, but possible variations along the molar mass distribution can also be measured in the case of heterogeneous multicomponent resins.
Figure 2: MMD and short chain branching (SCB) frequency measured by GPC for a bimodal HDPE. The density of each of the components (black numbers in the graph, given in g/cm3 units) was calculated from an average of the SCB frequency in each molar mass range.
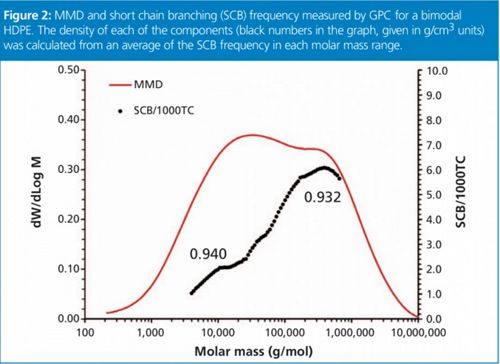
An example is provided in Figure 2 for a bimodal HDPE produced in a dual-reactor process. A lower molar mass high-density component is typically produced together with a second component of higher molar mass and a small amount of added comonomer. This balance results in enhanced mechanical properties, such as environmental stress cracking resistance (ESCR), for pipe applications. From a single GPC analysis, and in less than 1 h with minimum operator intervention, it is possible to obtain estimation of the density being produced in each of the two reactors, as well as the molar mass and the weight fraction of each component. The level of control on the process is thus greatly enhanced over the alternative methods based on bulk properties.
Conclusion
The control of the reactors for the manufacture of PE and PP is still based on measuring physical properties of the bulk material (Melt Flow Index, density), which gives quite limited information on the product itself. An enhanced and more refined control on the process and product can be realized by using more complete information given by the molar mass distribution (MMD).
A method specifically developed to measure the MMD in a manufacturing plant laboratory has been developed. It has been demonstrated that the total cycle time, including the sample dissolution step, can be reduced to around 30 min, well in line with the process control requirements.
Method reproducibility is good in terms of both average molar mass and polydispersity index (RSD around 0.5%). The detection of chemical composition (related to density) is provided by the built-in IR detector.
Alberto Ortín received his M.Sc in analytical chemistry from the University of Valencia, Spain. His Ph.D. in chemistry was focused on polyolefin characterization applying infrared (IR) detection to various separation techniques. He is currently a scientist at Polymer Char and his research interests lie predominantly in the implementation of new polyolefin characterization technologies and advanced detection methods.
Esther López is in the analytical department at Polymer Char.
Benjamín Monrabal is the R&D Director at Polymer Char.

ISC 2024: An Interview with Amarande Murisier
October 8th 2024As part of our ISC 2024 coverage, we recently interviewed Amarande Murisier of the University Hospital of Lausanne, Switzerland (CHUV) about her winning the Rising Stars of Separation Science Award for Biopharmaceutcal Analysis and her scientific background.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)