Measuring More from Less: The Evolution of Hyphenated and Combined Measurements
Special Issues
Jonathan Sweedler

One long-term goal of measurement science is to obtain greater chemical information from smaller samples in a faster, more robust manner. However, while the demands on analytical methods intensify, many of our measurement approaches have reached their practical limits of performance. Over the past few decades, one successful solution has been to link a number of individual techniques to measure the same sample, often achieving the goal of pooling the merits of each. Perhaps no other area has reaped the rewards of combined measurements as much as the separation sciences have.
Obviously, instrumental hyphenation is not new: It is appropriate to contemplate Tomas Hirschfeld’s thoughts, published almost 40 years ago in a special issue devoted to the topic of “hy-phen-ated methods” (1), wherein he stated: GC−MS, LC−MS, GC−IR, LC−IR, TLC−IR. . . . As the rising tide of alphabet soup threatens to drown us, it seems appropriate to look at the common denominator behind all of these new techniques, and those we have not heard from. The hyphen which is the single common constituent of all these acronyms is also the symbol of their common principle.
Hirschfeld anticipated that characterizing more-complex samples would require more-complex instrumental combinations, better separations, and smaller samples-certainly accurate predictions. Accordingly, the first stage of many hyphenated instrument platforms is a separation. The combination of liquid chromatography (LC) with chemical characterization approaches, including mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy, are now commonplace, as are combining separations and mass spectrometry with themselves multiple times (for example, multidimensional separations and multiple-stage mass spectrometry [MSn]).
A key objective of a hyphenated approach is to obtain a more complete chemical characterization of a sample. Often, spatial or temporal information is also needed, but is usually not acquired with a separation. It is partly to address this deficiency that combinations of hyphenated measurements on the same samples are increasingly being reported. An overarching goal is to increase the information content from the sample along new dimensions.
So, what hyphenated combinations are appearing? Among many possible directions, an important area of focus is advancing the ability to probe large numbers of individual cells for their activity and chemistry (2,3). Although gas chromatography (GC)−MS and two-dimensional (2D) electrophoresis characterizations of individual neurons were reported in the 1970s (4,5), recent approaches such as capillary electrophoresis (CE)−MS now allow greater proteome and metabolome coverage from selected individual cells (6,7), while direct matrix-assisted laser desorption−ionization (MALDI)−MS provides less comprehensive chemical information but from thousands of cells (8,9). Can such disparate measurement approaches be combined? Because a MALDI−MS measurement leaves a significant amount of sample behind, we recently demonstrated high-throughput single-cell MALDI profiling with the ability to perform CE−MS on selected cells (10). This workflow, illustrated in Figure 1, can be used to obtain MALDI−MS and CE−MS information from the same cells (Figure 2). The ability to perform detailed chemical assays on selected locations of a tissue after MALDI-MS highlights the importance of the order of measurement and sample compatibility. As a different example, several groups are combining vibrational spectroscopic imaging with MS imaging (11,12); these combinations are effective because at least one of the measurements leaves the sample behind for the next measurement.
Our single-cell metabolomics workflow combines direct MS profiling and CE-based MS in a two-tiered process. Image prepared by Troy Comi and Elizabeth Neumann from the Sweedler group at the University of Illinois at Urbana-Champaign.
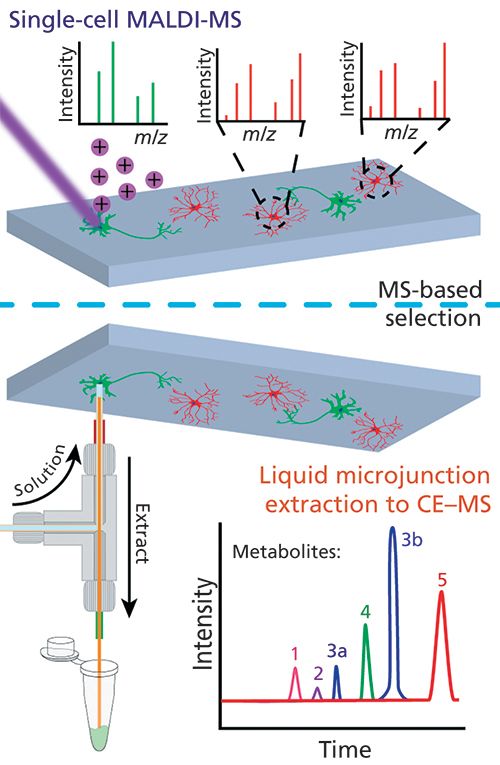
The combination of direct
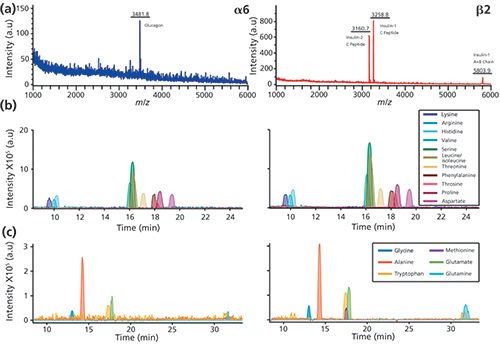
What does the future hold for these measurement combinations? As long as several of the measurements are mostly nondestructive, they can be used sequentially on the same sample. A potential, intriguing application I expect to see in the future--combining electrochemical imaging to monitor cellular release, followed by MS imaging and discrete chemical measurements via LC−MS or CE−MS-should provide a time, space, and chemical map from a living neuronal network.
The trend toward combining higher information content measurements will certainly continue. Regardless of the specific combinations used, separations hyphenated to MS will play prominent roles because of their ability to provide unmatched chemical information from complex samples. Expected advances in the coming decade will allow us to probe a living assembly of cells with the trio of chemical-spatial-temporal resolution as never before possible.
References
- T. Hirschfeld, Anal. Chem.52, 297A (1980).
- The National Institutes of Health Common Fund program on Single Cell Analysis. https://commonfund.nih.gov/singlecell/.
- The 2016 NSF sponsored workshop “Measuring the Brain: From the Synapse to Thought.” http://tinyurl.com/yag2935z.
- R. Ruchel, J. Chromatogr.132, 451−468 (1977).
- T.M. Liffe, D.J. McAdoo, C.B. Beyer, and B. Haber, J. Neurochem.28, 1037−1042 (1977).
- R.M. Onjiko, S.A. Moody, and P. Nemes, Proc. Natl. Acad. Sci. U.S.A. 112, 6545−6550 (2015).
- J.T. Aerts, K.R. Louis, S.R. Crandall, G. Govindaiah, C.L. Cox, and J.V. Sweedler, Anal. Chem. 86, 3203−3208 (2014).
- E.T. Jansson, T.J. Comi, S.S. Rubakhin, and J.V. Sweedler, ACS Chem. Biol. 11, 2588−2595 (2016).
- T.D. Do, T.J. Comi, S.J. Dunham, S.S. Rubakhin, and J.V. Sweedler, Anal. Chem.89, 3078−3086 (2017).
- T.J. Comi, M.A. Makurath, M.C. Philip, S.S. Rubakhin, and J.V. Sweedler, Anal. Chem.89, 7765−7772 (2017).
- T. Bocklitz, K. Bräutigam, A. Urbanek, F. Hoffmann, F. von Eggeling, G. Ernst, M. Schmitt, U. Schubert, O. Guntinas-Lichius, and J. Popp, Anal. Bioanal. Chem. 407, 7865−7783 (2015).
- D.R. Ahlf, R.N. Masyuko, A.B. Hummon, and P.W. Bohn, Analyst139, 4578−4585 (2014).
Jonathan Sweedler is the James R. Eiszner Family Endowed Chair in Chemistry and the director of the School of Chemical Sciences at the University of Illinois at Urbana-Champaign in Urbana, Illinois.

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)


















