Measuring Antibody Molecular Weight by SEC-MALS
The Application Notebook
Malvern Instruments Ltd.
A purified polyclonal antibody (IgG) is separated and fully characterized using the Viscotek SEC-MALS 20, allowing calculation of molecular weight and radius of gyration (Rg).
Therapeutic recombinant antibodies represent a growing proportion of biopharmaceuticals and are primarily classed as Immunoglobulin G (IgG). However, proteins have a tendency to aggregate over time and one challenge for biologic drugs is that the presence of aggregates will stimulate an immune response. Size-exclusion chromatography (SEC) is a powerful tool that is commonly used to look at the aggregation of proteins. While most SEC systems use a single concentration detector such as ultraviolet (UV), the addition of light scattering allows the molecular weight of the protein to be measured independent of its retention volume. The new SEC-MALS 20 detector, which uses multi-angle light scattering (MALS), is ideal for this application. In addition, the MALS detector makes it possible to measure the radius of gyration (Rg) of molecules that scatter light anisotropically.
In this application note, a purified polyclonal antibody (IgG) is separated using SEC and characterized using the Viscotek SEC-MALS 20.
Experimental Conditions
Samples were analyzed using a Viscotek TDAmax system connected to Viscotek SEC-MALS 20. The mobile phase was phosphate buffered saline, which was also used to prepare the IgG for analysis.
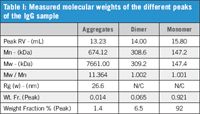
Table I: Measured molecular weights of the different peaks of the IgG sample
Results
The SEC-MALS results are presented in Table I. The monomer (15.80 mL) and dimer (14.00 mL) peaks are clearly identified by the measured molecular weights and low polydispersity (Mw/Mn). No size (Rg) can be measured for these peaks as they are below the isotropic scattering threshold of 10–15 nm. Studying Figure 1, it is just possible to see that the SEC-MALS 20 show the same response for the monomer peaks at all angles. The aggregate peak (13.23 mL) is clearly different. The molecular weight is higher and more polydisperse, which shows that there is a variable composition of molecules within the aggregate peak. Because it is large, the light scattering response varies with angle and can be used to measure the Rg.

Figure 1: Overlay of MALS detector responses for IgG.

Malvern Instruments Ltd.
Enigma Business Park, Grovewood Road, Malvern, UK
tel. +44 (0) 1684 892456
E-mail: salesinfo@malvern.com
Website: www.malvern.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.














