Analysis of Maleic Acid in Starch-Based Products Using a New Bonded Zwitterionic HILIC Column and Low UV Wavelength Detection
EMD Millipore
Organic acids are hydrophilic compounds with acidic properties where the carboxylic acids are predominant. Organic acids are generally weak acids that do not dissociate completely in water and they are present in every meal we eat. Organic acids are also used in food preservation because they can penetrate bacteria's cell wall and disrupt their normal physiology. Ion chromatography is the favored analytical technique for quantitative and qualitative purposes, but reversed-phase (RP) chromatography coupled to various detection techniques such as electrochemical, UV, RI, or MS is also common. To retain organic acids in reversed phase mode a requirement is to add ion-pairing reagents, work at low pH, and/or use completely aqueous mobile phases. Citric and tartaric acid are difficult to retain and resolve sufficiently in RP mode, and often there is co-elution of malic acid and succinic acid when using ion chromatography.
Hydrophilic interaction liquid chromatography (HILIC) has appeared and proven as an attractive technique for separation of small polar molecules such as organic acids. HILIC is considered as a MS friendly technique using volatile acetate or formate buffers in the mobile phase, conditions preventing analysis at low UV wavelength. However, bonded zwitterionic HILIC columns can be used with inorganic buffers like phosphate despite the limited solubility of potassium phosphate in high acetonitrile eluents. There are though some useful guidelines when using inorganic buffers (i.e. phosphate) in HILIC but the same also apply to RP when using a high proportion of acetonitrile in the eluent. Use premixed mobile phase and avoid pure acetonitrile as one mobile phase constituent. Precipitation of salt generally occur when using over 80 volume-% acetonitrile in the mobile phase, though at low buffer strengths 85% is the absolute maximum. In gradient mode, the difference between mobile phase A and B should be as small as possible and HILIC gradients should be shallower than in RP since changes in mobile phase composition has a larger effect in HILIC than in RP and thus require longer column equilibration.
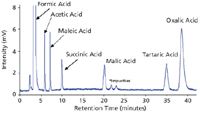
Figure 1: Separation of seven organic acids (20 μL injection) on a SeQuant® ZIC®-cHILIC (3 μm/100 à ), 250 à 4.6 mm column using a mobile phase consisting of 80:20 (v/v) acetonitrile and potassium phosphate 25 mM pH 6.0 (5 mM total ionic strength). The flow rate was 1.0 mL/min and the UV detector was set at 205 nm.
This application note shows that organic acids can be successfully analyzed with high sensitivity, low UV wavelength detection, using phosphate-based buffer systems and zwitterionic SeQuant® ZIC®-cHILIC columns, see Figure 1. A key characteristic of the ZIC®-cHILIC column in this separation is the controlled ionic interactions offered by its zwitterionic phosphorylcholine group orientation, which results in higher retention and thus allow the use of lower amounts of acetonitrile, fully compatible with the solubility levels of phosphate buffer. At the same time the ionic interactions between the organic acids and the ZIC®-cHILIC column are weak enough to not give excessive retention or poor selectivity dominated by extremely strong ionic interaction. Recently this column has proven useful in Taiwan for detecting maleic acid in tainted starch based products, see Figure 2.
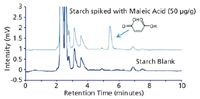
Figure 2: Chromatogram showing the analysis of a) starch sample and b) maleic acid spiked starch sample on a SeQuant® ZIC®-cHILIC (3 μm/100 à ), 250 à 4.6 mm column using a mobile phase consisting of 77:23 (v/v) acetonitrile and di-potassium hydrogen phosphate 20 mM pH 7.0 (4.6 mM total ionic strength). 20 μL samples (dissolved in mobile phase) were injected and analyzed at a flow rate of 1.0 mL/min using a UV detector set at 214 nm.

EMD Millipore.
290 Concord Rd., Billerica, MA 01821
tel. (800) 645-5476
Website: www.emdmillipore.com

Assessing Thorium-Peptide Interactions Using Hydrophilic Interaction Liquid Chromatography
February 4th 2025Paris-Saclay University scientists used hydrophilic interaction liquid chromatography (HILIC) coupled to electrospray ionization mass spectrometry (ESI-MS) and inductively coupled plasma mass spectrometry (ICP-MS) to assess thorium’s interaction with peptides.















