Maximizing HPLC Productivity with 2.5 μm Extended Performance (XP) Columns
The Application Notebook
The migration to chromatographic methods utilizing smaller particle sizes has been well demonstrated to increase productivity through faster, more efficient separations.
The migration to chromatographic methods utilizing smaller particle sizes has been well demonstrated to increase productivity through faster, more efficient separations. While significant improvements have been achieved with the use of sub-2 µm chemistries, the transition to UPLC® may be limited by available resources and regulatory guidelines (i.e., USP regulations). Here we demonstrate the increases in productivity attainable with conventional HPLC configurations, utilizing the new 2.5 µm, eXtended Performance (XP) columns.
Experimental Conditions
All data was collected on an Alliance® HPLC System with a PDA detector (254 nm). These evaluations utilized XSelect™ CSH™ C18, XSelect CSH™ C18XP and core-shell C18 columns of various particle sizes and dimensions. The mobile phases were 0.1% formic acid in water and 0.1% formic acid in acetonitrile. A linear gradient from 25% to 35% acetonitrile was used with gradient times and flow rates scaled according to particle size and column dimension. The sample is a mix of basic tricyclic antidepressants; nordoxepin, doxepin (cis & trans), desipramine, imipramine, nortriptyline, amitriptyline, and trimipramine (10 µg/mL each).
Method Transfer
Chromatographic methods can be scaled by maintaining the same ratio of column length to particle size (L/dp). A method developed for a 5 µm, 4.6 × 150 mm column easily transfers to a 4.6 × 75 mm column with a 2.5 µm particle size. This transfer represents an acceptable change in both column length and particle size under current USP guidelines (1). In addition to scaling for the shorter column, scaling the method to a faster flow rate (based on the smaller particle size) further shortens analysis time as demonstrated in Figure 1 (A and B) for the separation of tricyclic antidepressants on XSelect™ CSH C18 chemistries. This method transfer example yields similar peak capacity and resolution and provides a 70% reduction in analysis time.
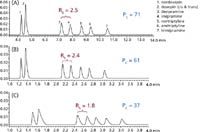
Figure 1: Gradient separations of tricyclic antidepressants on a 5 µm, 4.6 Ã150 mm, XSelect⢠CSH⢠C18 (A), a 2.5 µm, 4.6 à 75 mm, XSelect⢠CSH⢠C18 XP (B), and a 2.6 µm, 4.6 Ã75 mm, core-shell C18 column. Sample loading on each column was 144, 72, and 72 ng, respectively. Flow rates were 0.99, 1.97 and 1.97 mL/min, respectively. Each chromatogram has been notated with average peak capacity (PC) and resolution for the desipramine/imipramine pair.
Comparison with Core-shell Particles
Core-shell particle technology has shown utility in providing faster, more efficient separations. However, because the solid core is not chromatographically active, mass loading is limited for higher concentration samples. This is most dramatic for basic compounds under low ionic strength acidic conditions. This is demonstrated for the sample of basic tricyclic antidepressants on a core-shell C18 column in Figure 1(C). The mass load for each analyte on the 4.6 × 75 mm columns was 72 ng, a concentration typical for routine drug analysis.
Conclusions
The 2.5 µm XP columns provide the opportunity to scale existing HPLC methods to faster, more efficient methods while utilizing existing HPLC instrumentation and maintaining regulatory compliance. Packed with fully porous particles, better peak shapes are achieved for some applications at higher mass loads relative to core-shell particles. The XP columns, available in each of the 14 XBridge™ and XSelect™ column chemistries, provide a diverse range of selectivity choices while enabling full scalability between HPLC and UPLC.
For full application note, go to www.waters.com/720004271
Reference
(1) USP Chapter <621> Chromatography, USP34-NF29. The United States Pharmacopeial Convention, official from December 1, 2011.
©2012 Waters Corporation. Waters, XSelect, CSH, XBridge, UPLC, Alliance, and The Science of What's Possible are trademarks of Waters Corporation.
Waters Corporation
34 Maple Street Milford, MA
tel. (508) 478 2000, fax (508) 872 1990
Website: www.waters.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















