Analysis of "Broad Spectrum" UVA and UVB Components in Sun Care Products for Compliance with New FDA Regulations
The Application Notebook
The FDA has made changes to how products containing sunscreen are labeled in the U.S. to ensure they meet the new regulations set forth for safety and effectiveness. The new regulations will require companies that want to use the "broad spectrum" label to test for both UVA and UVB protection.
The FDA has made changes to how products containing sunscreen are labeled in the U.S. to ensure they meet the new regulations set forth for safety and effectiveness. The new regulations will require companies that want to use the "broad spectrum" label to test for both UVA and UVB protection. Previous regulations only dealt with preventing sunburn which is primarily due to UVB radiation but did not address UVA which protects against early aging and skin cancer. All products that claim to provide broad spectrum SPF protection are regulated as sunscreen products. Therefore, the regulations the FDA has developed for over the counter (OTC) sunscreen products apply to cosmetics, moisturizers, lip balms, and shampoos labeled with SPF values.

Table I: Experimental Conditions
Results
Conclusion
The methodology in Figure 1 provides one test method to determine both UVA and UVB compounds most commonly used in the sun care industry. Figure 2 is a sun care spray run with the same analytical conditions eluting six UVA/B compounds. All UVB and UVA compounds are eluted in a single run and can be quantitated in the ranges recommended by the FDA (SPF 15–50+). Only broad spectrum products with an SPF value of 15 or higher can claim to reduce the risk of skin cancer and early skin aging if used as directed with other sun protection measures.
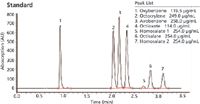
Figure 1: All UVA and UVB compounds are eluted in a single run using a Brownlee SPP column with an optimized PerkinElmer HPLC system
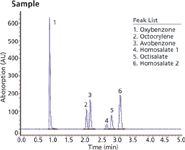
Figure 2: SPF 50 sun care spray shows elution of both UVA/B compounds.
References
(1) U.S. Food and Drug Administration, 2012. Information for Consumers, 2012. Retrieved from http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm258468.htm.
PerkinElmer
940 Winter Street, Waltham, MA 02451
Tel. (800) 762-4000, fax (203) 944-4904
Website: www.perkinelmer.com

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.
MilliporeSigma: Ultrapure Water for Sensitive LC-MS Analysis of Pesticides
March 25th 2025The aim of the study was to illustrate the efficiency of Milli-Q® water purification systems in eliminating pesticides from tap water, thereby producing and delivering reliable and consistent-quality ultrapure water suitable for pesticides analysis















