Analytical Methods to Characterize and Quantify PEG and PEGylated Biopharmaceuticals
The Application Notebook
Biotherapeutic peptides and proteins are often PEGylated (covalently bonded to polyethylene glycol polymers) to improve bioavailability, reduce immunogenicity, and extend circulating half-life (1). Achieving the desired properties for each application depends on optimizing the number and site of polymers attached, chain length, and the degree of chain branching.
Biotherapeutic peptides and proteins are often PEGylated (covalently bonded to polyethylene glycol polymers) to improve bioavailability, reduce immunogenicity, and extend circulating half-life (1). Achieving the desired properties for each application depends on optimizing the number and site of polymers attached, chain length, and the degree of chain branching. This is accomplished by careful choice of PEGylation reagents and reaction conditions. For example, the Thermo Scientific MS(PEG)n series consists of N-hydroxysuccinimide (NHS) esters that react at pH 7–9 with primary amine groups. The molar ratio of reagent-to-protein should be optimized for each application.
While the PEGylated protein products can be characterized by HPLC with UV or MS detection, it is difficult to quantify the PEG and PEGylation reagents because they lack a chromophore. In this work, poor UV sensitivity was overcome by using HPLC with a charged aerosol detector that responds to all nonvolatile analytes even in the absence of a chromophore. On-line 2D, LC combining size exclusion and reversed phase chromatography, provided automated analyte trapping, matrix removal, separation and detection. From a single injection, the high molecular weight PEGylated proteins were analyzed by size exclusion chromatography (SEC), while the low MW PEGylation reagent was trapped, separated from the buffer salts present in the reaction mixture, and analyzed by reversed phase chromatography. These methods and results are discussed in the poster and summarized here (2).
Experimental Conditions
System: Thermo Scientific Dionex UltiMate 3000 x2 Dual RSLC, Thermo Scientific Dionex Corona ultra RS Charged Aerosol Detector (CAD™ ).
Column: Thermo Scientific MAbPac SEC-1 5 µm, 300 Å, 4 × 300 mm
Corona™ ultra RS™ : Filter setting 4, Nebulizer Temp: 15 °C
Pump Right was used for the SEC analysis and ran isocratically with 10 mM ammonium acetate, pH 6.7 with 5% acetonitrile.
Pump Left was used for the reversed phase analysis. The mobile phases are (A) 100 mM ammonium acetate pH 4.7, (B) acetonitrile, and (C) deionized water. The timing of the gradient and loop fill is shown in the poster (2).
Results
Figure 1 shows the result of using 2D-LC-charged aerosol detection for varying molar excesses of the PEGylation reagent. The protein retention times shifts on the SEC column in the 1st dimension indicating higher molecular weight species are being formed at the higher MS(PEG)8 concentrations. This is also observed in the inset chromatogram with the peak monitored by UV at 280 nm. The retention time and area reproducibility of the residual PEGylation reagent was <0.1% and <1%, respectively, for three injections of the 5x MS(PEG)8 point with charged aerosol detection. The limit of detection for the MS(PEG)8 was determined to be <10 ng on column allowing for low level residual analysis to be accomplished with this method.
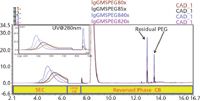
Figure 1: Overlay of 2D-LC analysis of IgG reacted with varying concentrations of MS(PEG)8 by charged aerosol dectection, (inset) UV @ 280 nm.
Conclusion
The Corona ultra RS detector can be used in combination with 2D LC to qualify and quantify PEGylated proteins, as well as residual PEGylation reagents and byproducts.
References
(1) S. Morar, J. Schrimsher, and M. Chavez, "PEGylation of Proteins: A Structural Approach" Bio Pharm. Int. April, 34–36 (2006).
(2) C. Crafts, B. Bailey, M. Plante, and I. Acworth, "Analytical Methods to Qualify and Quantify PEG and PEGylated Biopharmaceuticals," presented at the Pittsburgh Conference, Orlando, Florida, 2012.
Thermo Fisher Scientific
22 Alpha Road, Chelmsford, MA

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














