Material Identification by HPLC with Charged Aerosol Detection
The Application Notebook
Material identification is a common need in many industries, most notably for pharmaceutical manufacturing where the United States Pharmacopeial Convention (USP) defines many identification tests.
Material identification is a common need in many industries, most notably for pharmaceutical manufacturing where the United States Pharmacopeial Convention (USP) defines many identification tests. A new platform approach to material identification was developed using high performance liquid chromatography (HPLC) with charged aerosol detection (CAD). The method described in this article incorporates a mobile-phase gradient consistent with a hydrophilic interaction liquid chromatography (HILIC) separation mechanism and uses a mixed-mode column, which provides reversed-phase and cation- and anion-exchange properties. This technique provides a rapid and flexible alternative to USP <191>, and other analytical identification techniques. The chromatographic separation of 13 substances included in USP <191> has been demonstrated. Furthermore, simultaneous quantitation and impurity detection is achievable within the same analytical run.
Material identification is an integral part of the quality control process for chemical manufacturing and research, both of which need a generic, fast, and specific test method for a wide range of compounds. The typical practice in the pharmaceutical industry for raw material acceptance in manufacturing is minimally based on a vendor's "Certificate of Analysis," appearance and identification testing.
Identification testing of commonly used raw materials is often included in the United States Pharmacopeial Convention (USP). The USP sets quality and purity standards for products ranging from medicines to food ingredients that are enforceable in the United States by the Food and Drug Administration (FDA). USP chapter <191> (1) defines general identification tests. The materials described are identified by a distinctive set of tests based on their unique physiochemical properties. Identification of a single substance can range from the formation of a single precipitate to performing a flame test followed by a multistep precipitation and dissolving matrix. While these are acceptable methods for material identification in the pharmaceutical industry, many other technologies provide more rapid and generic capabilities for material identification.
Depending on the industry and application, material identification may be required for compounds from inorganic salts to heavy metals to organic solvents. Raw materials for pharmaceutical manufacturing do not contribute to the structure of the final active pharmaceutical ingredient (API) molecule; however, positive identification is generally required before use in the production process. Suitable identification techniques are selected based on unique material properties of the raw material of interest. Furthermore, confirmation of identification will be shown by comparison to a reference standard, which contains closely related substances (2). Not only will the reference standard confirm a positive identification, but also show that a positive response is not obtained from the closely related substances or possible interfering materials.
Many technologies are currently used for identification. These include, but are not limited to, Fourier transform infrared spectroscopy (FT-IR), ion chromatography (IC), pKa determination, and Raman spectroscopy. Technologies such as these are often limited by their inability to analyze multiple substances at the same time or within the same analytical run, and each technique is limited in the set of substances that it can detect. FT-IR lacks the ability to detect substances like inorganic salts or solutions in water, IC cannot detect neutral compounds, pKa determination cannot distinguish between acids with similar pKa values, and identification by Raman spectroscopy fails for compounds that fluoresce.
Charged aerosol detection (CAD) is a universal detection method for nonvolatile compounds or substances (3). CAD nebulizes eluent from the high performance liquid chromatography (HPLC) analysis to form particles. Ionized nitrogen gas then charges the particles and those charges are detected by an electrometer. Using CAD as a tool for identification allows us to take advantage of its inherent property as a universal detection method and detect significantly more substances than similar identification technologies or techniques.
The purpose of this work was to show that HPLC with CAD can be used for material identification. The identification includes, but is not limited to, many materials listed in USP <191>, which describes material identification for many alkali metals, halides, heavy metals, and anionic polyatomic species like sulfate.
Experimental
Reagents and Materials
The materials used for this work were all obtained from Sigma-Aldrich and were ACS grade or better. The materials used include sodium sulfate, sodium chloride, sodium phosphate, sodium nitrate, lithium hydroxide, magnesium lactate, sodium citrate, potassium phosphate, and ammonium formate for HPLC mobile phase.
Deionized water (>18.2 MΩ) was purified from a Milli-Q water purification system (Millipore). HPLC-grade acetonitrile was purchased from Burdick and Jackson.
Instrumentation
The chromatographic system was an Agilent 1260 HPLC system (Agilent) equipped with an on-line degasser, a quaternary pump, an autosampler, a column thermostat, a diode-array UV detector, and a Corona Plus CAD detector (ESA Inc., A Dionex Company). The column was a 50 mm × 3.0 mm, 3-μm dp Thermo Scientific Acclaim Trinity P1.
Results and Discussion
HPLC–CAD Method Development
Method specificity is a critical parameter for any identification method or technique (2). Separation-based method identification typically uses retention time of the sample compared to a standard; for instance, identification by HPLC–UV is commonly used. Our aim is to have a generic identification method, thus 13 common substances used in pharmaceutical manufacturing that are typically identification tested per USP <191> were selected: sodium, potassium, lithium, chloride, bromide, iodide, nitrate, sulfate, tartrate, calcium, phosphate, citrate, and magnesium. The method development was based on a method we published previously for ion analysis, in which 11 of these substances were separated using a mixed-mode column and hydrophilic interaction liquid chromatography (HILIC) conditions (4). The addition of bromide and lithium was necessary to provide additional coverage for the most common raw materials used in pharmaceutical manufacturing. Thus, there were three areas of focus for method development. These include adding lithium and bromide to the analysis, decreasing overall analysis time, and maintaining specificity by making gradient modifications.
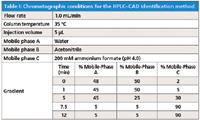
Table I: Chromatographic conditions for the HPLCâCAD identification method
The most desirable conditions satisfying all method development goals were achieved by increasing the flow rate to 1 mL/min, decreasing the starting acetonitrile to 50% while maintaining 2% 200 mM ammonium formate at the starting conditions for 1 min, and using a 7.5 min gradient. Table I shows the final method chromatographic conditions. Specificity was maintained as shown in Figure 1 despite gradient modifications. Furthermore, a reduction in analysis time was achieved, from 20 min to 12 min.
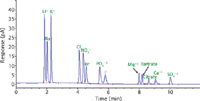
Figure 1: Chromatogram showing 13 substances in USP separated using the conditions in Table I.
Identification by HPLC–CAD
Identification by HPLC–CAD has many advantages compared to other techniques. One primary advantage of this technique is the rapid identification of a substance, such as potassium phosphate shown in Figure 2. For example, a previously prepared standard is run confirming the retention times of 13 substances, as shown in Figure 1. A solution of the sample (potassium phosphate), prepared at about 0.1 mg/mL, is injected after the standard. By comparison of retention times, potassium phosphate is easily identified. With each standard and sample injection taking less than 20 min, depending on equilibration time, the identification takes less than 40 min. Where this application excels is its ability to identify multiple substances within the same analytical run. For example, the analysis time for four different compounds, or seven substances (sodium chloride, potassium nitrate, lithium bromide, citric acid) would take less than 100 min, illustrating not only the application's diversity but also its efficiency.
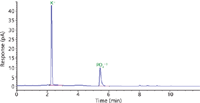
Figure 2: Chromatogram of potassium phosphate using the conditions in Table I.
Adding to the efficiency of the HPLC–CAD analysis is the ease of system setup and sample or standard preparation. For this specific application, all samples were prepared at 0.1 mg/mL in 80:20 (v/v) water–acetonitrile. Two approaches can be taken for standard preparation. Approach one is to prepare a standard, which represents only the substances to be identified. A second approach would be to prepare a stock solution of multiple substances as used in the potassium phosphate example. The stock standard would then be stored for future use, thus additional time can be saved. For both approaches, the standards are prepared quickly from commercially purchased standards such as 1000 ppm chloride standard for IC.
Identification by HPLC–CAD Versus USP <191>
Identification by HPLC–CAD has many advantages when compared to USP <191>. As shown in Figure 3, the identification of potassium and phosphate using USP <191> uses a large number of reagents. Some reagents can be purchased commercially whereas others may need to be freshly prepared. For example, to identify potassium and phosphate, eight reagents are required. In addition, equipment for a flame test is required for potassium identification. After an analyst has acquired all reagents, equipment, and glassware, the identification can be started. In total, nine steps are required to identify both substances and this would take significantly longer than the same identification by HPLC–CAD, which requires approximately 2 h of analyst time. A comparison of the required analyst time for identifying four compounds by HPLC–CAD as described above exhibits a larger time advantage. Using the USP <191> method for each substance, an analyst would spend many hours identifying each, whereas the analyst time commitment using HPLC–CAD would be no more than 1 h.
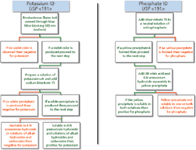
Figure 3: Flow chart depicting steps necessary to identify potassium and phosphate per USP .
Furthermore, if multiple lots of the same substance need to be identified per USP <191>, then each would require the same amount of steps and nearly double the analyst time commitment. In comparison, if HPLC–CAD was used, samples from each additional lot would be prepared in about 5 min and added to the same analysis.
One advantage that USP <191> possesses is the ability to identify substances such as ammonium, that are too volatile for detection using CAD. CAD cannot detect highly volatile substances because particles cannot be formed when leaving the nebulizer.
Identification by HPLC–CAD Versus Other Technologies
Other technologies are also available for identification of substances. Identification by IC has few, if any, advantages over HPLC–CAD. Performing an identification on an IC system will require only highly purified water, a column for retaining anions or cations, and an eluent generator, such as hydroxide. For a single substance like phosphate, this technique is quick and requires little analyst time. However, to identify phosphate, three instrument modifications are needed, including switching the eluent generator to methanesulfonic acid, changing to a cation retaining column, and switching the capillary connections between the detector and the negative ion suppressor to the detector and the positive ion suppressor. Compared to an HPLC–CAD technique, traditional IC techniques have no advantage because they cannot detect anions and cations simultaneously without instrument modification and a second analysis. IC also cannot detect uncharged substances, and although the method presented in this paper only describes charged substances, the CAD can also detect uncharged substances when combined with the right chromatographic conditions.
Simultaneous detection of anions and cations is possible by chelating the positively charged ions with the ligand EDTA, then an IC setup in anion mode can detect the anion and precomplexed cation EDTA ligand (5). Although this technique achieves some similarities to the HPLC–CAD technique, using it for multiple substances would require extensive method development. Other IC techniques are available including multieluent systems, in-series columns, zwitteric ion-exchange columns, and multidetector systems, making multi-substance detection possible (6). Unfortunately, these options include columns that are not commercially available and have complex instrumentation configurations, and uncommon mobile phases.
Infrared spectroscopy is a common technique for the identification of substances, including those in the pharmaceutical industry. Although FT-IR can be used for identification of many substances by comparing them to a reference standard spectrum, inorganic salts like sodium chloride and potassium bromide are infrared-transmitting substances and are therefore not susceptible to identification. Substances in water, such as 1 M citric acid, would be difficult to identify due to large interference from water.
Potentiometric titration could be used to identify acids such as fumaric acid and tartaric acid by their pKavalues. However, fumaric acid and tartaric acid have nearly identical pKa1 and pKa2 values (3.03 and 4.38 for fumaric acid, 3.02 and 4.36 for tartaric acid), so identification cannot be confirmed.
Raman spectroscopy can be used to identify substances such as manganese sulfate. A noninvasive technique using spatially offset Raman spectroscopy, offers quick scanning for identification (7). However, substance identification is limited due to fluorescence, which obscures the Raman spectra. Although multiple technologies are available to identify substances, all of them can only be used to identify a narrow set of compounds compared to HPLC–CAD.
Additional Advantages of HPLC–CAD
Although the goal of this article is to show how HPLC–CAD can be used for substance identification, the HPLC–CAD combination has additional applications (4) that can be performed simultaneously. One application is the ability to perform substance quantification. For example, using current chromatographic conditions, chloride has been shown to be linear from 0.4 μg/mL to 30 μg/mL and all 13 substances have similar linear ranges. Figure 4a shows the linearity, injection precision, and limit of detection (LOD) method qualification results that would satisfy regulatory requirements for assay methods. Method sensitivity is typically not required for a material identification method, but generally, CAD can achieve detection to the single-digit nanogram range. Detailed information regarding CAD sensitivity can be found in the literature (4). A simple linear fit with a dynamic range of two orders of magnitude was used here in Figure 4a, and it is adequate for assay method requirements. If a wider dynamic range is desired, a quadratic plot or linear log–log plot (8) can be used, as shown in Figures 4b and 4c, respectively, where the dynamic range of up to four orders of magnitude can be achieved.
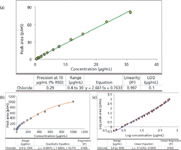
Figure 4: Method qualification results for the determination of chloride concentration with (a) simple linear regression; (b) quadratic regression; (c) logâlog plot linear regression.
Another advantage is the ability to collect impurity data, which again can be collected simultaneously. Because CAD is a universal detection method, impurities present in a sample are most likely to be observed. A major impurity in potassium phosphate is sulfate, which, as shown in Figure 5, is observed along with sodium. If a standard, as shown in Figure 2, is prepared quantitatively, then the amount of impurities can be determined. Thus, in one sample and analysis, the identity of potassium phosphate was confirmed and the purity of the sample was ascertained.
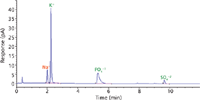
Figure 5: Identification of a potassium phosphate sample showing sodium and sulfate contamination.
The optimized method is not limited to only 13 substances. The method can be modified to include additional substances, either from USP <191> or not, depending on future identification needs.
Limitations of HPLC–CAD for Material Identification
As demonstrated above, HPLC–CAD for material identification based on the retention time of samples and standards provides significant practical advantages, especially for raw material identification in which the sample matrix is typically simple. However, CAD response is generally mass dependent and not spectral or physicochemical property dependent. Although this is an advantage for universal detection, it lacks specific structural information. For samples with a complex matrix or an unknown mixture in which definitive identification of impurities is needed, orthogonal detection methods or specific techniques such as mass spectrometry should be used.
Another limitation as mentioned earlier is that CAD cannot detect volatile compounds, such as ammonium acetate and formate. On the other hand, this is why ammonium acetate and formate are good mobile-phase modifiers for HPLC–CAD.
Conclusions
A new generic approach for material identification has been shown using a combination of an HPLC, CAD, and a mixed-mode column. Current industry standards in the pharmaceutical industry rely on USP <191> for material identification. However, our approach is a rapid and concise way for replacing many sections of USP <191> for material identification. Furthermore, this approach is a practical alternative for material identification in other industries or applications. Together with identification, the technique can simultaneously perform quantitation and detect impurities. However, for structural information, specific detectors, such as mass spectrometers, should be used.
Acknowledgments
We thank Sigrid Hubbell (Genentech) for manuscript review and helpful suggestions.
Brandon Scott, Kelly Zhang, and Larry Wigman are with Small Molecule Pharmaceutical Science, at Genentech Inc., in South San Francisco, California. Direct correspondence to: zhang.kelly@gene.com
References
(1) General Chapter <191> "Chemical Tests and Assays, Identification Tests — General" in United States Pharmacopeia 35–National Formulary 30 (United States Pharmacopeial Convention, Rockville, Maryland, 2012).
(2) International Conference on Harmonizaiton (ICH) Guidelines. Validation of Analytical Procedures Q2 (R1), Nov. 2005.
(3) S. Almeling, D. Ilko, and U. Holzgrabe, J. Pharm. Biomed. Anal. 69, 50–63 (2012).
(4) K. Zhang , L. Dai, and N.P. Chetwyn, J. Chromatogr., A 1217(37), 5776–5784 (2010).
(5) M. Bruzzoniti, E. Mentasti, and C. Sarzanini, Anal. Chim. Acta 382, 291–299 (1999).
(6) P. Nesterenko, TrAC, Trends Anal. Chem. 20, 311–319 (2001).
(7) M. Bloomfield, D. Andrews, P. Loeffen, C. Tombling, T. York, and P. Matousek, J. Pharm. Biomed. Anal. 76, 65–69 (2013).
(8) T. Vehovec and A. Obreza, J. Chromatogr., A 1217(10), 1549–1556 (2010).

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)

















