The LCGC Blog: Automated Dynamic Liquid–Liquid Microextraction (DLLME)—Improve Sample Extraction, Buy Some Time, and Reduce Environmental Footprint
The improved performance of chromatographic detectors, most notably mass spectrometers (MS), has enabled many advances in analytical science, however, one such advance may be given less prominence than perhaps it should.
Photo Credit: Prathum/stock.adobe.com

The improved performance of chromatographic detectors, most notably mass spectrometers (MS), has enabled many advances in analytical science, however, one such advance may be given less prominence than perhaps it should.
Typically, the volumes of liquid handled by automation “robots” are limited. Depending on the sample preparation application, this limitation in sample volume, alongside speed with liquids, can be handled. The achievable sample enrichment factors mean that smallerâscale liquid handling robots have been excluded from many important areas of analysis.
That has now changed significantly. We need to consider the positive implications that this may have on the quality, safety, and efficacy of our analytical measurement.
In the past decade, the sensitivity of mass spectrometric detectors (for example, triple quadrupole [QqQ] systems) has improved vastly, and an order of magnitude or greater increase in sensitivity has been achieved. Instrument manufacturers demonstrate subâfemtogram instrument detection limits (IDL) for gas chromatography–mass spectrometry (GC–MS) systems, and even single quadrupole GC–MS instruments are now capable of IDLs in the singleâdigit femtogram range. Triple quadrupole detectors used with high performance liquid chromatography (HPLC) that have electrospray atmospheric pressure ionization sources are stated to have IDLs in sub 100 femtogram with several claiming limits of half as much.
How Does this Enable the Use of Fully Automated, Online Sample Preparation Systems to Perform Trace Analysis?
The sample volume for typical, online sample handling robotics is usually less than 50 mL, because of the way in which syringes or low-volume pipettes are used for sample transfer and manipulation. This affects the speed with which this can be achieved. The instrument needs to achieve either a very high sample enrichment factor or very low detection limits. The combination of the improvement in mass spectrometric detector sensitivity with the introduction of extraction techniques-primarily the soâcalled microextraction techniques-means that sample preparation is now possible using automated online systems. Previously, this could only be achieved using traditional liquid–liquid or large volume solid-phase extraction (SPE).
So Why is This Important to Us?
Anyone who has laboured with manual sample extraction using shake flask techniques will know that these are fraught with issues, including the following:
- Large volumes of organic solvent are typically required, which is both environmentally damaging and a health hazard to the operator
- Attendant dangers of flask droppage or spillage
- Manual shaking or even shaking with an orbital mixer produces variable results.
The extracting solution needs to be preâconcentrated prior to injection, and this stage is highly prone to contamination and irreproducibility.
Automated, online sample preparation is capable of overcoming all of these disadvantages and achieving very low detection limits with only limited sample volumes. When operated in parallel processing mode, it can reduce the required sample preparation time to below the duty cycle time of the chromatographic system-improving throughput alongside the benefits of improved reproducibility. The benefits of improved accuracy, reproducibility, and reduced contamination are often associated with automated sample preparation.
Due to the increased detector selectivity associated with MS detection, where analyte signals may be selectively extracted using mass filtering, a general move towards simplification of sample preparation operations has been found. The number of applications published that use simple liquid–liquid extraction has markedly increased.
One such microextraction technique that is rapidly gaining in popularity is dynamic liquid–liquid microextraction (DLLME). The principle of the technique (which is distinct from traditional liquid–liquid extraction approaches), the facile sample processing paradigm, and the extraction and enrichment benefits are very impressive.
DLLME uses a small volume of immiscible extracting solvent to form an emulsion of micro-droplets within the sample. This presents a very high surface area for analytes to partition into. In order to increase the “contact” between the two phases, a disperser solvent is used, which is highly miscible with both phases and serves to reduce the interfacial tension between the sample and extracting solvent. After addition of the extracting and disperser solvents, the sample is vigorously mixed or vortexed to affect extraction. Importantly, due to the very high surface area contact, the time taken to reach equilibrium (maximum extraction) is relatively short. Following extraction, the sample is vortexed and the extracting solvent aspirated for subsequent analysis.
DLLME is used with aqueous samples and an organic extracting solvent. However, it is also possible with a slight adaptation to the approach, to extract analytes from organic samples with aqueous solvents. Crucially, the technique can achieve high enrichment factors as only a relatively small volume of extracting solvent is used and an increase in analyte concentration of 10–50× is typical.
A schematic representation of the DLLME process is shown in Figure 1.
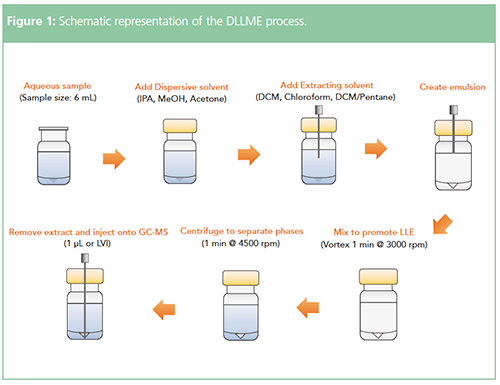
Implementation of automated online DLLME has required the development of several novel robotic tools that are worthy of further note. In order to rapidly transfer fairly large amounts of sample, pipetting tools are required to achieve sample transfer in reasonable time frames. Vortex mixing is crucial in order to affect speedy and reproducible extractions, and therefore, an automated vial vortexing tool is required. The development of a solventâsafe automated centrifuge is required to break the emulsion and form extractant sediment within the well of a high-recovery vial (where the extractant is denser than the sample). The final injection solution is aspirated from here.
Typically, the automated DLLME process can be performed in a comparable timescale to the chromatographic run time. With batch preparation or parallel processing modes, the sample preparation time for multiple samples is considerably shorter than the combined chromatographic analysis time for all samples.
This high-speed aspect is also important when considering method development and optimization. The factors typically considered in this stage might include:
- The volume of sample (1–30 mL*)
- Nature** of volume of extractant (100–500 μL)
- Nature*** and volume of disperser (5–10× extracting solvent volume)
- Sample pH adjustment (to promote extraction from aqueous to organic solvents, pH may be adjusted to reduce the degree of ionization of target analytes)
- Effects of salt addition (increasing the electrolytic strength of an aqueous sample can help promote the partition of polar analytes from an aqueous phase into an organic extractant)
- Vortex or mixing (extraction) speed and time (typically a few seconds to a few minutes)
- Injection volume (note that large volume injection GC can be used to improve detection limits even further, with most modern GC system having the ability to injection up to 1000 μL of the sample).
Notes:
* The volumes shown above are typical but will vary depending upon the sample type, desired degree of enrichment etc.
** The full range of aqueous immiscible organic solvents are available to use as the extracting solution.
*** Typical disperser solvents include isopropanol, acetone, methanol, acetonitrile, and dichlorobenzene.
Due to the very fast sample extraction, it is possible to optimize the DLLME procedure using a one-factor-at-a-time (OFAT) approach. Several publications cite the use of this experimental design to more efficiently arrive at the optimum experimental conditions. A simple example is shown in Figure 2.
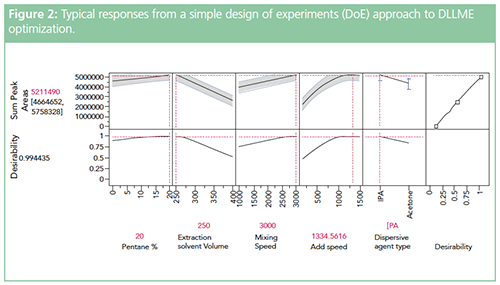
Alongside the parameters considered in Figure 2, length of extraction time was not found to be a significant factor in the range 0.6 s to 5 min, indicating, for this application, the high speed and the efficiency of the DLLME technique.
Figure 3 shows the MRM chromatogram and calibration curve produced following DLLME sample preparation of aldrin as part of a suite analysis of organochlorine pesticides (OCPs) and polychrorinated biphenyls (PCBs) from surface water, for which the figures of merit achieved are also shown.
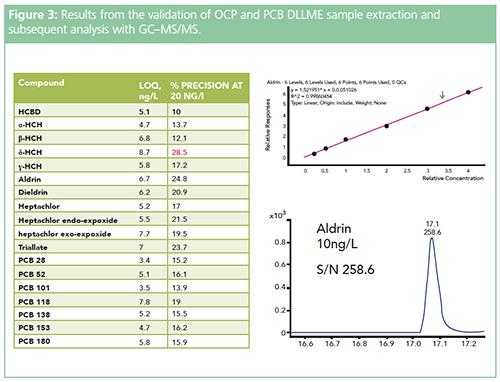
Despite the judicious choice of extracting solvent, sample pH, and electrolytic strength optimization, the fact remains that liquid–liquid extraction is not a highly selective sample preparation technique. However, as mentioned above, the use of the more highly selective triple quadrupole mass spectrometric detectors can help to mitigate this. It is also possible to combine the use of DLLME with more highly selective μSPE or molecularly imprinted sorbent (MIP) extraction, where the benefits of semiâtargeted sample enrichment and the more highly selective micro-sorbent extraction can also be combined in a rapid and automated fashion to produce highly selective and concentrated sample extracts. This may be an advantage when using LC–MS/MS techniques.
The DLLME process is versatile and can be applied to any situation in which liquid–liquid extraction is typically used. It can transform a manual and time-consuming sample preparation method into a rapid, sensitive, and fully automated online technique. To help justify the rather bold statements made in the title of this piece, Figure 4 shows a graphical representation of some proposed advantages of the DLLME technique for an application based on a manual liquid–liquid extraction using 50 mL of DCM and 400 μL for the automated method.
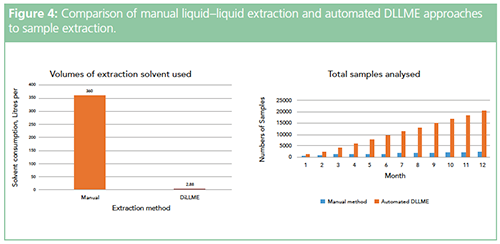
Automated DLLME is a technique that can exploit the improvements in mass spectrometric sensitivity and microextraction techniques to deliver benefits in speed, quality, and efficacy of sample extraction and enrichment. Significant solvent reduction, in comparison to traditional approaches, can positively impact the environmental footprint.
Tony Taylor is Group Technical Director of Crawford Scientific Group and CHROMacademy. His background is in pharmaceutical R&D and polymer chemistry, but he has spent the past 20 years in training and consulting, working with Crawford Scientific Group clients to ensure they attain the very best analytical science possible. He has trained and consulted with thousands of analytical chemists globally and is passionate about professional development in separation science, developing CHROMacademy as a means to provide high quality online education to analytical chemists. His current research interests include HPLC column selectivity codification, advanced automated sample preparation, and LC–MS and GC–MS for materials characterization, especially in the field of extractables and leachables analysis.
E-mail:tony@crawfordscientific.comWebsite:www.chromatographyonline.com

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





