Tips & Tricks GPC/SEC: Silica Versus Polymer‑Based Columns
The Column
The chemistry of samples analyzed using gel permeation chromatography/size-exclusion chromatography/gel filtration chromatography (GPC/SEC/GFC) is very diverse. Different chemistries of stationary phases are required to allow for true size separation. Several types of materials are available, all of which have their advantages and limitations. While silica‑based stationary phases are most common in high performance liquid chromatography (HPLC), for macromolecules polymer-based phases are popular.
gerda1201/stock.adobe.com

The chemistry of samples analyzed using gel permeation chromatography/size-exclusion chromatography/gel filtration chromatography (GPC/SEC/GFC) is very diverse. Different chemistries of stationary phases are required to allow for true size separation. Several types of materials are available, all of which have their advantages and limitations. While silicaâbased stationary phases are most common in high performance liquid chromatography (HPLC), for macromolecules polymer-based phases are popular.
Gel permeation chromatography/sizeâexclusion chromatography/gel filtration chromatography (GPC/SEC/GFC) users face the challenge of selecting the optimum stationary phase for their applications. The expectations regarding the column performance are high. GPC/SEC columns should provide:
- High resolution over a wide molar mass separation range
- Good stability (mechanical, chemical, thermal) and high solvent compatibility
- Minimal shear degradation and hindrance to diffusion
- Minimal or no interaction between stationary phase and sample.
Very often these expectations cannot be fulfilled equally. For example, a high resolution over a wide molar mass range can be achieved by using column banks. However, this increases the overall back pressure and hence the mechanical stability (column lifetime) can be reduced. Adjusting the conditions by adding modifiers or by setting a specific pH, so that no or minimal interactions are present, can also influence the chemical stability.
An understanding of the basic differences of typical stationary phase materials can help predict which expectations can be easily met and which are hard to achieve with a specific material.
Stationary Phases in GPC/SEC
Typical stationary phases in liquid chromatography (LC) are rigid porous silica particles (inorganic packings) and soft or semi-rigid (highly cross-linked) organic gel particles (1). Each of the packings has its advantages and limitations.
Soft organic gel packings are mainly used for preparative separations of large waterâsoluble macromolecules such as DNA, sugars, or proteins. The aim of the analysis is to achieve pure fractions. These materials are available in large particle sizes only, often above 45 µm, and are extremely sensitive to high pressures.
Detailed characterization and determination of the molar mass distribution is done using semi-rigid highly cross-linked organic polymer particles. The higher degree of cross-linking allows for much higher pressure stability. Therefore small particles can be synthesized and be efficiently packed to allow for the highest resolution. While soft organic gels can also be obtained in bulk, semi-rigid crossâlinked gels are delivered already packed in columns with metal bodies. For some applications the metal body is coated so that metal ions will not influence the separation or disturb the detection. Highly cross-linked polymer gels are synthesized with very different chemistries. Their polarities are optimized to match the polarities of samples and solvents (mobile phases).
By far the highest pressure stability is exhibited by rigid inorganic silica particles packed in columns. Again the goal of the analysis is a high performance separation to allow the determination of the molar mass distribution. As a result of the high pressure stability, very small particles can be synthesized. Different chemical modifications are available to match the different chemistries of the analytes involved, for example, the silanol groups present on the silica packing surface can be modified. Typical modifications are trimethylsilane for use in polymer separations and diol for separation of proteins and peptides in aqueous solutions.
Based on their chemistry, specific organic gels and silica particles are often assigned to either i) the analysis of water-soluble samples in water or buffer solutions or ii) to use with organic mobile phases.
For some organic gels the use of the wrong mobile phase (for example, water instead of an organic mobile phase) can destroy the packed bed of the column making it useless for separation. Others, such as polyester copolymer networks or specific silica phases, can tolerate water and organic solvents. However, this does not imply that the columns should be used because interaction-free chromatography can only be achieved for balanced polarities of mobile phase, sample, and stationary phase (2).
Advantages of Silica Materials
Faster and Easier Solvent Change: Silicaâbased phases offer a faster solvent exchange. Polymer-based columns often need long equilibration times when being transferred from one mobile phase to another, while silica-based materials need less time to (re-)equilibrate. Also, due to their significantly higher pressure stability, silica columns require less care during the change from one mobile phase to another.
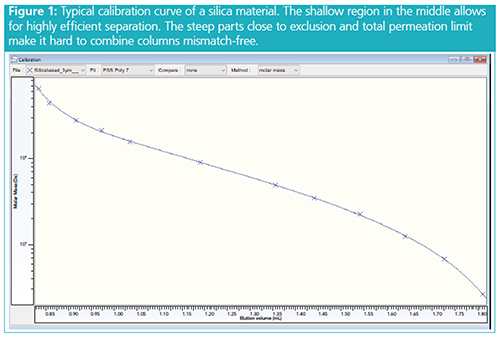
High Resolution in a Narrow Molar Mass Separation Range: Compared to highly cross-linked organic gel materials, silica-based materials often exhibit calibration curves with a very shallow slope in a specific molar mass region. This results in excellent resolution for this region. Figure 1 shows a calibration curve of a silica material. Silica-based materials are therefore ideal for applications that require only separation of narrowly distributed samples, for example, for protein aggregation/stability studies.
Advantages of Polymer Gel Materials
Broader Separation Range Achievable: Figure 1 also shows that calibration curves of silica materials often become very steep close to the exclusion and total permeation limit. In GPC/SEC, columns of different pore sizes are often combined to increase the molar mass separation range. Steep parts and sharp curvatures in calibration curves make it harder to combine columns and the risk for pore size mismatch is dramatically enhanced (3). Thus, it is
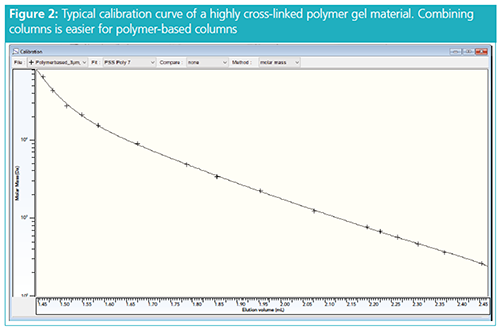
much easier to combine polymer gel columns than silica columns. Polymer gel columns show shallower slopes and curvatures when approaching the limits (compare Figure 2). Very large molar mass separation ranges with still sufficient resolution and lower risk for chromatographic artifacts can therefore be accomplished when using highly crossâlinked organic particles.
Temperature Stability: Highly crossâlinked polymer particles exhibit better temperature stability than silica materials. This is particularly important when GPC/SEC has to be performed in high viscous solvents or when polyolefins have to be analyzed. Polyolefins require temperatures > 160 °C to be kept in solution. Highly viscous solvents require a higher temperature to reduce the solvent viscosity and to enhance mass transfer (and thus the resolution). The column lifetime of polymer-based columns for such applications is much longer because polymer-based materials can easily tolerate temperatures above 90 °C, even for long times.
pH Stability: Polymer-based columns can tolerate a very wide pH range, while silicaâbased columns are often only (longâterm) stable in a limited range of 2–8. For all applications that require a more extreme pH range polymer-based columns are the better choice. Typically, the materials are stable between 2 and 13. However, there are also columns that, although they can chemically tolerate the pH range of 2–13, are best operated in a specific range, for example, to analyze polycations (pH 2–7) or polyanions (pH 7–13). The stability over a wide pH range also means that different reagents can be used to clean and regenerate the columns after extensive use. This is particularly useful for prep-scale chromatography.
Higher Purity for Advanced Detection: Multi detection is very common in GPC/SEC. Hyphenation, for example, with light scattering or viscometry, is standard in many laboratories, and combining with mass spectrometry (MS) is also becoming more popular. Advanced detectors, such as light scattering detectors, are very sensitive to particle shedding (4). Mass spectrometers are sensitive to metal ions. Polymer-based materials can often be obtained in a quality that allows for trouble-free detection. Metal ions, which disturb MS detection, are not present and pre-equilibration of columns for light scattering detection is available for many materials.
Less Interactions: Last, but definitely not least, polymer-based columns are important because they are less prone to interactions. Unreacted silanol groups on the silica surface can lead to interactions between sample and stationary phase, something that definitely should be avoided in size-exclusion chromatography. Even optimized mobile phases with additives and modifiers can sometimes not fully suppress interactions.
The presence of interactions should be investigated when one or more of the following observations are made:
- no elution
- late elution after the system peak
- unexpected very high resolution
- peak tailing
- shifting retention times or elution volumes
- low reproducibility of retention times or areas.
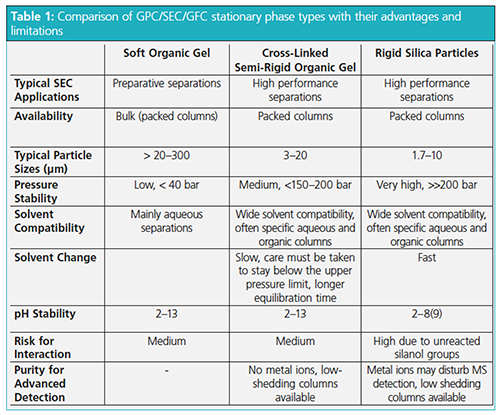
Table 1 compares and summarizes the advantages and limitations of the different materials.
Conclusions
Soft organic gel particles are available in bulk or packed in glass columns. They are used for separation of water-soluble high molar mass samples at low or medium pressure.
Molar mass determination is done with rigid porous silica materials (inorganic) and cross-linked organic gel materials available in packed metal columns or specific bioinert hardware.
Silica-based columns offer higher pressure stability and an excellent resolution in a narrow molar mass range.
Polymer-based columns have a wider pH and temperature stability, are less prone to interactions, and can be easily combined to cover a wider molar mass range.
References
- A.M. Striegel, J.J. Kirkland, W.W. Yau, and D.D. Bly, Modern Size-exclusion Liquid Chromatography, 2nd edition (John Wiley & Sons, Inc., New Jersey, USA, 2009).
- T. Hofe, The Column3(12), (2007).
- T. Hofe, The Column4(4), (2008).
- T. Hofe and M. Susewind, The Column15(4), 14–19 (2019).
Daniela Held studied polymer chemistry in Mainz, Germany, and works in the PSS software and instrument department. She is also responsible for education and customer training.
Kirsten Oleschko studied polymer chemistry in Mainz, Germany, and works in the PSS production department. She is responsible for synthesis of particles and column technology.
E-mail: DHeld@pss-polymer.comWebsite: www.pss-polymer.com

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Rethinking Chromatography Workflows with AI and Machine Learning
April 1st 2025Interest in applying artificial intelligence (AI) and machine learning (ML) to chromatography is greater than ever. In this article, we discuss data-related barriers to accomplishing this goal and how rethinking chromatography data systems can overcome them.
Influence of Concentration in Conventional GPC/SEC and Advanced Detection GPC/SEC
March 21st 2025Sample concentration is a parameter that can influence the quality of gel permeation chromatography/size-exclusion chromatography (GPC/SEC) separations and the obtained results. Understanding this influence can help to support the development of reliable GPC/SEC methods.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)






