Investigating the Environmental Impact of Organotins
Organotin compounds are widely used in a variety of industries for their antibacterial and fungicidal properties (1–3). While they have a broader range of technological applications than the organic compounds of other metals (4), there are concerns surrounding their use due to environmental pollution considerations and potential toxicity to mammals. This is because some organotins are known to be harmful to aquatic organisms, while others may act as immunotoxins (5), or as endocrine disruptors that affect reproduction (5–9).
Organotin compounds—also known as tin organics and stannanes—are comprised of tin bonded to hydrocarbon substituents. They are classified as mono-, di-, tri-, and tetra-substituted, according to the number of organic groups they contain (9) (Figure 1). Organotin compounds serve different purposes depending on the extent of substitution. Tetra-substituted organotins—such as tetrabutyltin, tetraoctyltin, and tetraphenyltin—can be used as starting materials or catalysts. They Norway are ineffective as biocides, and relatively non-toxic (10).
Tri-substituted organotins have fungicidal and bactericidal properties (1–3,10), conferred by the organic groups attached. Triphenyltins, for example, are typically used in antifouling paints—a practice now banned internationally—and as agricultural fungicides, as well as miticides and acaricides (10). Tributyltins are industrial biocides that have been used in antifouling paints and in wood treatments and preservatives. They are also used as disinfectants and molluscicides, and as antifungal agents in textiles and industrial water systems, such as cooling tower and refrigeration water systems. They can even be used for the control of the tropical disease schistosomiasis.
Di-substituted organotins—for example, dibutyltin and dioctyltin—are used as catalysts in the silicone curing and production of polyurethane foam, and as polyvinyl chloride (PVC) heat stabilizers. Similarly, mono-substituted organotins— such as methyltin, butyltin, octyltin, and monoestertins—are employed as PVC heat stabilizers (8–9).
The European Union permits the use of 2-ethylhexyl 10-ethyl-4,4-dioctyl-7-oxo- 8-oxa-3,5-dithia-4-stannatetradecanoate (DOTE) as an additive in the manufacture of plastic food-contact materials and articles, subject to certain restrictions (11). MOTE (2-ethylhexyl 10-ethyl-4-[[2-[(2-ethylhexyl) oxy]-2-oxoethyl]thio]-4-octyl-7-oxo-8- oxa-3,5-dithia-4-stannatetradecanoate) is used as a polyurethane catalyst andplastic stabilizer (12).
Origins of Organotins
Organotins are typically found in PVC products, water pipes, food wrap, polyurethane coatings, polyester, plastic trims, textiles, leather, screen prints, biocides, and pesticides, as well as inks and marine antifouling paints (5,8,9). They may also be found in silicone, due to their use as catalysts in the production process (9).
Environmental Impact of Organotins
Concerns around the persistence, bioaccumulation, and toxicity of organotins have led countries across the world to introduce legislation restricting their use. Tetraorganotins, although relatively non‑toxic, can slowly decompose or metabolize to tri-substituted compounds (10)—such as tributylin— which are considered the most toxic organotin variants (8). They may induce immunological impairment (5,13), act as neurotoxins—inducing behavioural abnormalities, as well as being toxic to the developing central nervous system— or as endocrine disrupters that disturb steroid biosynthesis and degradation, as well as affecting reproduction (5–6). Dioctyltin derivatives have also been listed as substances of very high concern due to their toxicity towards reproduction (14). Tributyltin and its degradation products— dibutyltin and monobutyltin—remain in marine sediments for many years, and trialkyltins in general are known to be toxic to aquatic life. In addition, the primary source of human exposure to organotin compounds is seafood (13). Consequently, their use in marine antifouling paints is prohibited because the residues present an environmental and health hazard (1–3,6). Food and potable water can also suffer from organotin contamination from industrial effluents, and via leaching from PVC water pipes (15). Compounds such as dibutyltin and tributyltin may cause skin irritation and eye irritation (9). DOTE— which is not manufactured, imported, or marketed as a mono-constituent substance, but only in reaction mass with MOTE (16)— is proposed for inclusion in the REACH Authorization List (Annex XIV of the REACH Regulation), as both compounds are toxic to reproduction (11,16,17).
Regulations and Restrictions
The global use of organotins is governed by a number of regulations and restrictions. European regulations include REACH annex XVII, REACH Restricted Substance List 2021 (18), and the REACH Candidate List (14,17). Under REACH annex XVII, organostannic compounds (entry 20) (4) dibutyltin, dioctyltin, and tri-substituted organotin compounds are restricted to 0.1% tin by weight when used as antifouling agents in paints. Similarly, organotin compounds included in the REACH Candidate List are restricted to 1% by weight.
Elsewhere, the use of organotins is governed by country-specific regulations, including:
- Japan (9):
Act on Control of Household Products Containing Harmful Substances (Act No. 112 of 1973) (19)
- South Korea (9):
Safety/Quality Labeling Act (KC) (20)
Self-Regulatory Safety Confirmation Act (21)
- Australia:
Workplace exposure standards for airborne contaminants (2019) regulations (22)
Australian Water Quality Guidelines for Fresh and Marine Waters (ANZECC, 1992) (23).
Analytical Challenges
Stability: Most organotins are stable in neat form although some, such as tri‑n‑octyltin chloride, may form precipitates on storage. However, they are considered unstable in solution—particularly some monochlorides—where they may decompose into unknown degradation products or, under certain conditions, the position of the alkyl substitutions may change. Consequently, ISO methods recommend that organotin solutions are used for no more than three to 12 months after preparation, and are stored in a cold, dark environment (24,25).
Purity: While many commercially available organotins claim to have high purity, this quality often falls well below the specifications stated. Products promoted as pure compounds may also contain other organotins that are likely to be co‑analyzed, resulting in incorrect results. Most commercially sourced products require extensive purification before they can be released as reference materials, and in some instances, the only route to reliable and high purity reference materials is to synthesize the required compounds. Purity is assessed by derivatization with a Grignard reagent, followed by analysis by gas chromatography (GC), quantitative nuclear magnetic resonance (qNMR), and other relevant methods, including direct liquid chromatography–mass spectrometry (LC–MS)
Multiple Stable Isotopes: Mass spectrometry analysis is complicated by the fact that tin has 10 stable isotopes—more than any other element. 120Sn is the most abundant isotope, accounting for almost a third of all tin, although 118Sn and 116Sn are also common. As a result, the analysis of mixes of organotin compounds can lead to quite complex spectra.
Analysis of Organotins
Most organotin analysis is associated with the determination of levels in soils and sediments in rivers and harbours, as specified in ISO 17353:2004 Water quality—Determination of Selected Organotin Compounds—Gas Chromatographic Method (24) and ISO 23161.2:2018 Soil quality—Determination of Selected Organotin Compounds—Gas chromatographic Method (25). These are complemented by the more recent ISO 22744.1:2020 Textiles and Textile Products— Determination of Organotin Compounds— Part 1: Derivatization Method Using Gas Chromatography (26) and ISO 22744.2:2020 Textiles and textile products—Determination of Organotin Compounds—Part 2: Direct Method using Liquid Chromatography (27).
GC and GC–MS quantification of organotin compounds is based on alkylation of organotin chlorides to form tetrasubstituted ethyl derivatives (1):
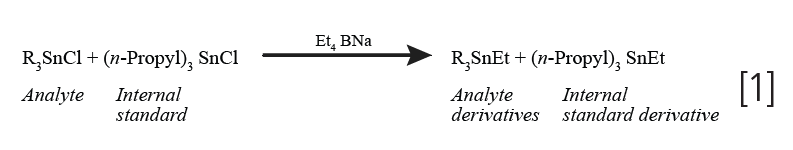
Commonly employed internal standards include:
- Tri-substituted organotin compounds that are not present in industrial products, typically tripropyltin chloride and triheptyltin chloride
- Deuterated compounds
- Trialkyltin chlorides based on one stable tin isotope.
The US EPA Validated Test Method 8323-01: Determination of Organotins by Micro-Liquid Chromatography-Electrospray Ion Trap Mass Spectrometry (28) offers an alternative approach. This method describes the use of solid-phase extraction discs, solvent extractions (for biological tissues), and micro-liquid chromatography (μLC) coupled with electrospray ion trap mass spectrometry (ES-ITMS) for the determination of organotins (as the cation) in waters and biological tissues.
Alternative Antifouling Agents
The ban on the use of organotins such as tributyltin chloride as antifouling agents has been a great success, and they have now been replaced by copper and zinc compounds and organics, for example, tralopyril (29) and dichlorooctylisothiazolinone (DCOIT) (30). However, robust environmental risk assessments on substitute agents are required to fully evaluate the risk.
Conclusions
Organotin compounds have wide-ranging applications; however, their use is not without risk to human health and the environment. In many countries, regulations are in place to monitor and control their use to help mitigate these harms. Analysis is required to assess compliance with restrictions, but this is subject to complications, such as low and incorrectly declared purity of standards, cross-contamination, and instability in solution. Care should be taken to select appropriate references to ensure the accuracy of conclusions drawn from the data collected.
References
- Chiron, BMF 9 – Organotin analysis: http:// www.chiron.no/GetFile.ashx?id=120
- Chiron, BMF 24 – Deuterated Alkyl- and Aryltin compounds: http://www.chiron.no/GetFile. ashx?id=126
- Chiron, BMF 41 – Deuterated Tin Chlorides: http://www.chiron.no/GetFile.ashx?id=136
- D. Ghazi, Z. Rasheed, and E. Yousif, Arc. Org. Inorg. Chem. Sci. 3(3), 344–352 (2018).
- AFIRM Group, Chemical Information Document Version 1.0, January 2018, Organotin compounds: https://www.afirm-group.com/ wp-content/uploads/2018/01/afirm_organotin_ compounds.pdf
- Y. Kotake, Biol. Pharm. Bull. 35(11), 1876–1880 (2012).
- European Commission Scientific Committee on Health and Environmental Risks, Revised Assessment of the Risks to Health and the Environment Associated with the Use of the Four Organotin Compounds: TBT, DBT, DOT and TPT, (2006): http://ec.europa.eu/health/ph_risk/ committees/04_scher/docs/scher_o_047.pdf
- Get the facts: organotins: https://saferchemicals. org/get-the-facts/toxic-chemicals/organotins Accessed 9 December 2021.
- Technical guidance on organotin compounds: https://www.tuvsud.com/en/e-ssentials-newsletter/past-topics/technical-guidance-on-organotin-compounds Accessed 9 December 2021.
- Organo-tin compounds: http://www.npi.gov. au/resource/organo-tin-compounds Accessed 9 December 2021.
- BPA and DOTE Among Substances Proposed for Authorization under REACH: https://www. packaginglaw.com/news/bpa-and-dote-among-substances-proposed-authorization-under-reach.
- MOTE – Monooctyltin tris- Ethylhexylthioglycolate Polyurethane Catalyst and Plastic Stabilizer: BNT – MOTE – Monooctyltin tris-Ethylhexylthioglycolate | BNT-chemicals. Accessed 28 March 2022.
- S. Das, in Product Safety and Restricted Substances in Apparel, S. Das, Ed. (Woodhead Publishing India, 2013), pp.14–28.
- European Chemicals Agency, Candidate List of Substances of Very High Concern for Authorization (published in accordance with Article 59(10) of the REACH Regulation): https:// echa.europa.eu/web/guest/candidate-list-table Accessed 9 December 2021.
- N.J. Snoeij, A.H. Penninks, and W. Seinen, Environ. Res. 44, 335–353 (1987).
- European Chemicals Agency, Background document for 2-ethylhexyl 10-ethyl-4,4-dioctyl- 7-oxo-8-oxa-3,5-dithia-4-stannatetradecanoate (DOTE): https://echa.europa.eu/ documents/10162/e74fee6c-6a4b-d848-a712- 6c7c0244a5b7
- Chiron, BMF 87 – Substances of Very High Concern: http://www.chiron.no/en/documentation/newsletters/environmental/bmf- 87-substances-of-very-high-concern-svhc
- REACH Annex XVII: REACH Restricted Substance List 2021: REACH Annex XVII: REACH Restricted Substance List 2021 (chemsafetypro. com) Accessed 9 December 2021.
- Act on Control of Household Products Containing Harmful Substances (Act No. 112 of 1973): http://www.japaneselawtranslation. go.jp/law/il/?printID=&ft=1&re=2&dn=1&x=47&y=16&co=01&ia=03&ja=04&ky=act+on+control+of+household+products+containing+harmful+substances&page=1&vm=02 Accessed 9 December 2021.
- Safety/Quality Labeling Act (KC): https://www. katri.re.kr/en/contentsid/778/index.do Accessed 9 December 2021.
- Self-Regulatory Safety Confirmation Act (KC): https://www.katri.re.kr/en/contentsid/779/index.do Accessed 9 December 2021.
- Safe Work Australia, Workplace exposure standards for airborne contaminants (2019): https://www.safeworkaustralia.gov.au/system/ files/documents/1912/workplace-exposure-standards-airborne-contaminants.pdf Accessed 9 December 2021.
- Australian Water Quality Guidelines for Fresh and Marine Waters: https://www.waterquality. gov.au/sites/default/files/documents/ANZECC- 1992-guidelines.pdf Accessed 9 December 2021.
- ISO 17353:2004 Water Quality — Determination of selected organotin compounds — Gas chromatographic method: https://www.iso.org/standard/31362.html Accessed 14 February 2022.
- ISO 23161.2:2018 Soil Quality — Determination of selected organotin compounds – Gas chromatographic method: https://www.iso.org/ standard/73990.html Accessed 14 February 2022.
- ISO 22744.1:2020, Textiles and textile products — Determination of organotin compounds — Part 1: Derivatization method using gas chromatography: https://www.iso.org/standard/75067.html Accessed 14 February 2022.
- ISO 22744.2:2020, Textiles and textile products — Determination of organotin compounds — Part 2: Direct method using liquid chromatography: https://www.iso.org/standard/73789.html Accessed 14 February 2022.
- United States Environmental Protection Agency, Validated Test Method 8323: Determination of Organotins by Micro-Liquid Chromatography- Electrospray Ion Trap Mass Spectrometry: https://www.epa.gov/hw-sw846/validated-test-method-8323-determination-organotins-micro-liquid-chromatography
- Chiron, Tralopyril - a potential new antifouling biocide of concern: http://www.chiron.no/ GetFile.ashx?id=10882
30. V. Silva, C. Silva, P. Soares, E.M. Garrido, F. Borges, and J. Garrido, Molecules 25(4), 991 (2020).
Jenny Button is Co-owner and Deputy Director of the reference material producer, Chiron AS. She is a forensic toxicologist with 15+ years of experience in the field, including 13 years as head of forensic toxicology at St George’s University of London. Jenny has held several leading roles within sales and marketing including as product specialist at LGC Standards and director/country manager at the German rapid test producer Nal-von-Minden. She draws heavily on her own experiences to provide a practical and customer‑focused approach to her position.

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)















