Lowering Limits of Quantitation in LC–MS/MS Analyses for Food Safety and Environmental Applications
Technological advances in tandem quadrupole mass spectrometry (MS) are improving reliability, sensitivity, and quantitative analysis, as well as providing the lower limits of quantitation necessary for food safety and environmental applications. Regulatory requirements updates have consistently required enhanced sensitivity across the board, thereby creating challenges for laboratories—especially with difficult-to-analyze negative ionizing compounds. In addition, updated instruments are reducing laboratory operating costs and the environmental impact of mass spectrometry (MS) systems, including savings on energy and gas usage, as well as lowering heat and audible noise output.
Quantitative analysis using tandem quadrupole mass spectrometry (MS) is essential for many applications in food safety and environmental analysis. However, constantly evolving regulatory requirements for the analysis of food safety and toxic contaminants in the environment have required lower levels of detection at ecologically damaging levels. Improvements in the performance of the mass spectrometer for reliable and sensitive ion detection are critical for many laboratories.
At the same time, laboratories are actively working to address environmental sustainability in tandem with the need to continually manage operational costs. Among these needs are lower electricity and gas consumption, as well as a reduction in the heat output of instruments to lessen the burden on facility heating and cooling systems. The rising cost of energy has resulted in organizations looking for new ways to minimize their energy usage and environmental impact, while still obtaining the lower levels of quantitation needed. New mass spectrometer instrument designs are working to achieve both goals. The following experiments describe the use of a tandem quadrupole mass spectrometer with a photomultiplier detector to determine the impact on both sensitivity and overall laboratory operations for food safety and environmental applications.
Food Safety
Routine analysis of anionic polar pesticides has become a requirement for many food safety laboratories. Previous polar pesticide approaches were typically a series of selective single residue methods, which required significant effort for the analysis. The introduction of the Quick Polar Pesticides (QuPPe) method has allowed the analysis of foodstuffs for highly polar pesticides not amenable to common multi‑residue methods (1). Here it was examined if the need for lower limits of quantification in anionic polar pesticide analysis could be addressed with the enhanced negative ion sensitivity of a tandem quadrupole mass spectrometer in combination with a photomultiplier detector (2).
Results indicated the ability to achieve limits of detection in the low and even sub‑μg/kg region and it could be combined with a generic extraction such as the QuPPe method to bring a multi-residue approach to the analysis. The linear response range for the anionic polar pesticides was tested over the range of 0.5–200 μg/kg (0.25–100 ng/ mL in vial concentration) for cucumber matrix and 2–200 μg/kg (0.25–25 ng/ mL in vial concentration) for wheat flour matrix. The limit of quantification (LOQ) was defined as the lowest calibration standard in these calibration sequences: 0.5 μg/kg for cucumber matrix and 2 μg/kg for wheat flour matrix. Internal standards were used in the calibration assessment for all compounds (except ethephon). In all cases the residuals for calibration were <20% and correlation of determination (r2) values were all 0.99 or greater. Example calibrations from cucumber and wheat flour matrix standards are demonstrated in Figure 1.
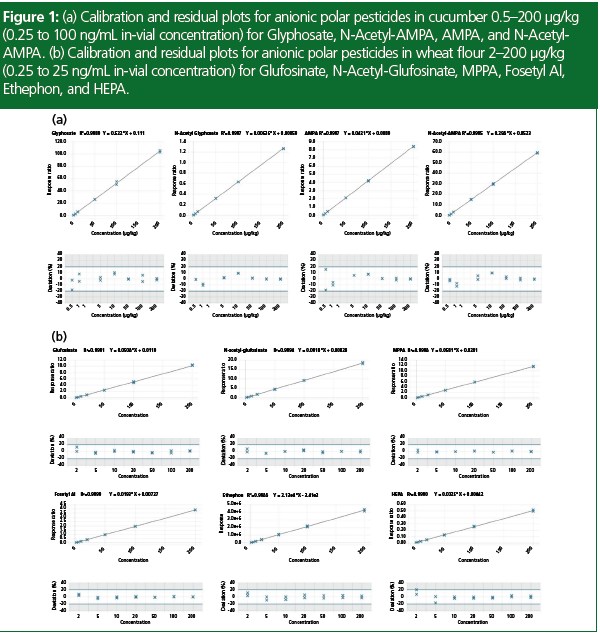
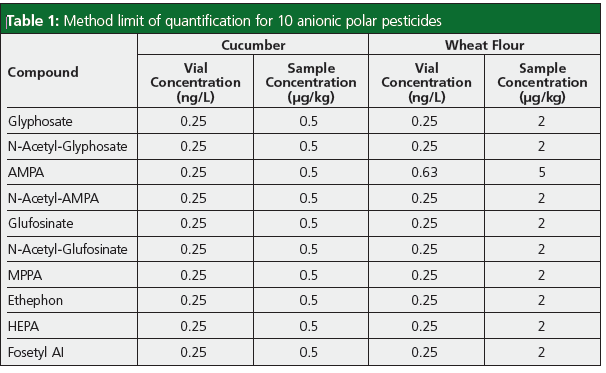
From the calibration experiments the method LOQ was calculated as the lowest calibration standard where the quantifier and qualifier transition were detected. Those limits are listed in Table 1. The difference in sample LOQs observed between the different sample types is attributable to the different dilution factor within the QuPPe v12 extraction procedure for “wet” commodities (cucumber) versus “dry” commodities (wheat flour). The in-vial concentrations that were detected were 0.25 ng/mL for all anionic polar pesticides studied for both commodities except for AMPA, which had a slightly higher in-vial concentration of 0.63 ng/mL in wheat flour matrix and is attributed to signal suppression from the matrix.
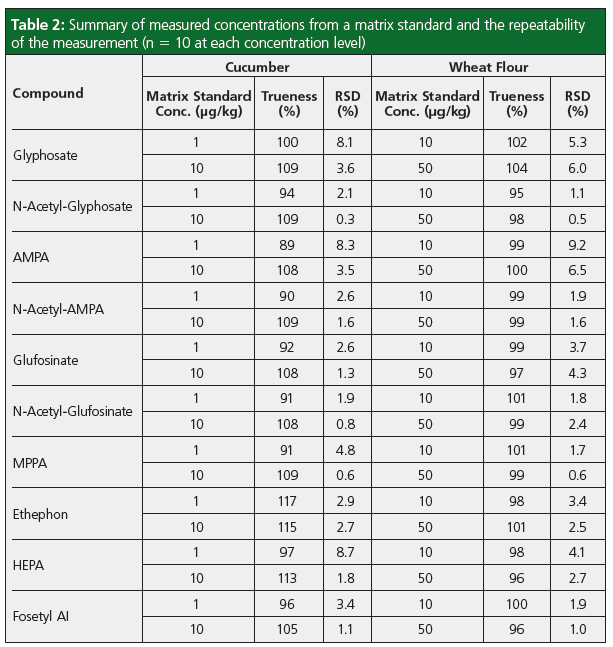
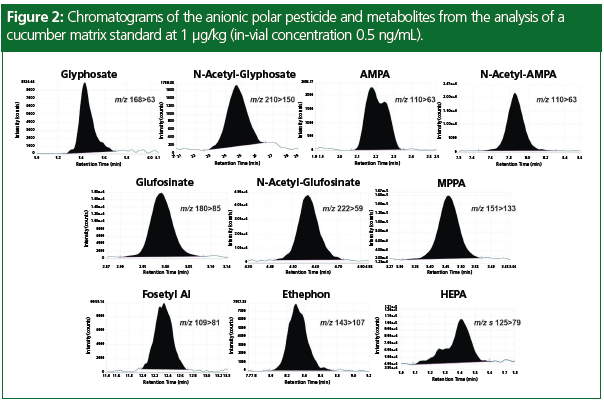
Trueness and repeatability for the analysis of the polar pesticides were assessed for both cucumber and wheat flour matrices by repeatedly injecting a matrix standard and quantifying the response against a calibration graph generated from bracketed calibration standards. Table 2 displays the results from these experiments and demonstrates that the instrument is capable of accurately quantifying residues of anionic polar pesticides at concentrations of 1 μg/ kg in cucumber (a representative vegetable matrix) and at 2 μg/kg in wheat flour (a representative cereal matrix), with AMPA slightly higher in wheat flour at 5 μg/kg. Example chromatograms for the anionic polar pesticides in cucumber matrix at 1 μg/ kg are displayed in Figure 2.
The results indicate the enhanced sensitivity in negative ionization mode, which makes achieving significantly lower levels of quantification possible. This has resulted in achievable limits of quantification for the anionic polar pesticides in cucumber (representative vegetable sample) of 0.5 μg/ kg and 2 μg/kg for all but AMPA—where 5 μg/kg was achieved—in the wheat flour (representative cereal sample).
Environmental Contaminants
Per-and polyfluoroalkyl substances (PFAS) are a group of chemicals used to make fluoropolymer coatings and products that resist heat, oil, stains, grease, and water. PFAS have made their way into public drinking water supplies, with estimates that over 200 million Americans have tap water that is contaminated. Testing for PFAS is an incredible challenge and laboratories must be ready to meet demands for sample throughput, trace detection, and complex matrices, all while keeping pace with regulations.
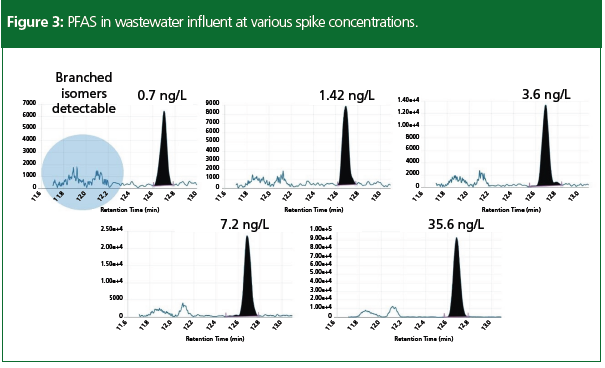
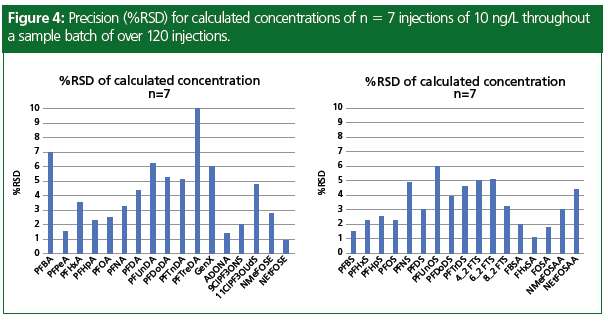
A method detection limit (MDL) study was performed following the EPA procedure EPA 821-R-16-006 using 10 replicates in reagent water (3). The sensitivity of the MS system with a photomultiplier tube (PMT) detector is shown in Figure 3, with five different levels of PFAS spiked into wastewater samples. Both the branched and linear isomers were detectable at the lowest spike level, allowing for accurate quantitation of all isomers in the sample even near the detection limits. Figure 4 demonstrates the stability of the method performance in the 10 ng/L continuing calibration verification (CCV) sample that was injected seven times throughout the sample batch of approximately 120 samples. The precision of the calculated concentrations was within 10% relative standard deviation (RSD) for all compounds in the method, with many at 5%. The concentrations of the PFAS detected in the four water samples are listed in Table 3.

Further Sustainability Considerations
Laboratories that conduct analyses of food safety and toxic contaminants in the environment have related concerns about their own internal sustainability. When considering tandem quadrupole mass spectrometry instruments, it’s widely viewed that higher sensitivity is better. While manufacturers may develop increasingly sensitive instruments, they are faced with making compromises elsewhere. For example, widening the entrance to the mass spectrometry analyzer and providing enhanced pumping to cope with the increased gas load can increase sensitivity, but it introduces more matrix into the system, which increases the need for cleaning and, as a result, downtime. In addition, the enhanced pumping capacity increases the electricity consumption of the instrument, the overhead costs for customers, and is not as environmentally sustainable.
Conclusion
Analytical laboratories face many challenges with performance and productivity, and confidence that analytical instrumentation can keep pace is critical. Scientists must continuously adapt new technology to reach the lower limits of quantitation required by regulatory agencies, particularly for challenging negative ionizing compounds. Technology advancements in mass spectrometry instrumentation have been shown to deliver lower limits of quantitation to meet these requirements, with up to 15× more sensitivity for challenging negative ionizing compounds than previous instruments.
As the regulatory landscape continues to evolve, the need to deliver consistent robustness and reproducibility will only increase as laboratories work to maintain performance and productivity over a longer period of time. Detection limits will continue to decrease as regulatory agencies move to protect consumers from contaminates in food, water, and the environment. At the same time, analytical laboratories will need to obtain these lower levels of quantification while also finding ways to reduce their energy usage and environmental impact. Advances in mass spectrometry instrumentation can help to meet both of these goals.
References
- M. Anastassiades, A.-K. Wachtler, D.I. Kolberg, E. Eichhorn, H. Marks, A. Benkenstein, S. Zechmann, D. Mack, C. Wildgrube, A. Barth, I. Sigalov, S. Görlich, D. Dörk, and G. Cerchia, Quick Method for the Analysis of Highly Polar Pesticides in Food Involving Extraction with Acidified Methanol and LC– or IC–MS/ MS Measurement - I. Food of Plant Origin (QuPPe‑PO-Method) –Version 12 (published on EURL-SRM website on 23 July 2021): https://www.eurl-pesticides. eu/docs/public/tmplt_article. asp?CntID=887&LabID=200&Lang=EN
- S. Adams, G. Barknowitz, and K.L. Organtini, Detection of Anionic Polar Pesticides in Food Samples Using the Xevo TQ Absolute With Sub μg/kg Limits of Quantification (Waters Application Note, 720007567, March 2022).
- K.L. Organtini and S. Adams, Improved Sensitivity for the Detection of Per- and Polyfluorinated Alkyl Substances in Environmental Water Samples Using a Direct Injection Approach on Xevo TQ Absolute (Waters Application Note, 720007559, March 2022).
Mark Roberts is Principle Product Manager at Waters, responsible for a series of tandem quadrupole mass spectrometers, and has 17+ years’ experience in product and segment marketing. Mark obtained his Ph.D. in analytical chemistry from Leeds University, UK.
E-mail: Mark_Roberts@waters.com

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
















