Comparison of PFAS Sample Preparation Between WAX + Dispersive GCB and WAX/GCB Cartridges from Water and Soil Extracts Per Draft EPA 1633
The recent draft of EPA Method 1633 specifies the use of a polymeric weak anion exchange (WAX) SPE sorbent in combination with graphitized carbon black (GCB) sorbent for the cleanup of solid samples, soils, biota, sediments, or non-drinking water samples. This procedure adds time to the cleanup step and presents the opportunity for loss of analytes and introduction of imprecision. This article describes a significant improvement to the guidelines wherein the two sorbents are contained within a single tube, offering the opportunity for decreased sample processing time and equivalent accuracy and precision.
Polyfluoroalkyl substances (PFAS) are a group of manufactured chemicals that have been used in industry and consumer products since the 1940s. There are estimated to be >6000 possible forms of PFAS compounds, but most have not been manufactured. The most studied and characterized are perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) (1), which have been replaced with other PFAS in the United States in recent years. The EPA has developed Draft Method 1633, “Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC–MS/MS” (2) to assist in PFAS analysis (3). This method was designed as an EPA 1600 series method. The DOD later released QSM 5.4, which had QA criteria specific for 1633. This method involves a two-step solid-phase extraction (SPE) approach using weak anion exchange (WAX) plus a separate graphitized carbon black (GCB) cleanup using a GCB powder, known as dispersive GCB (dGCB). For water samples, the dGCB powder is added after extraction. The purpose of the additional GCB cleanup step is to eliminate matrix that can cause interference and to reduce bias. Limitations of using dGCB are well known as stated in Draft EPA Method 1633, “it is important to minimize the time the sample extract is in contact with the carbon.” Besides these practical limitations, adding dGCB is very labour-intensive and is therefore not ideal due to the added time needed to add, mix, and centrifuge the dGCB for each sample, especially in high‑throughput laboratories. Cartridges were therefore developed as a single cartridge stacked with WAX and GCB sorbents that function as a traditional SPE cartridge with a built‑in polishing step to meet the method guidelines. These cartridges reduce the need for multiple extraction tubes when compared with the current draft method. The utility of WAX and GCB in a stacked SPE format for EPA 533 and 537.1 for a variety of water matrices has previously been demonstrated (4,5). The goal of this study was to validate method performance for the broader compound list in EPA 1633 and demonstrate the same utility for water and soil extracts. Figure 1 shows the SPE cartridges used for this comparison study.
As a guidance method, EPA 1633 makes provisions to demonstrate equivalency, “… a laboratory is permitted certain options to improve separations or lower the costs of measurements. These options include alternative extraction, concentration, and cleanup procedures, and changes in sample volumes, columns, and detectors” (2). In order to demonstrate equivalency, the Initial Precision and Recovery (IPR) as described in EPA 1633 section 9.2.1 was used for the comparison studies for water and soil matrices. For the water extract comparisons, 500 mL of reagent water for each sample was used for four samples. For the soil comparison study, one batch of 5 g of Ottawa sand was spiked and extracted. The extraction panel was expanded by adding three additional analytes to reflect the California Water Board panel for PFAS compounds in wastewater discharges (6).
Comparison of Water Extracts
For water samples, the precision of the extractions was determined by comparing the percentage relative standard deviation (%RSD) of spiked native PFAS analytes between cartridges A, B, and C shown in Table 1. Water samples were extracted using two different procedures. Samples were either extracted with WAX followed by dGCB powder as described in EPA 1633 or extracted using the dual stacked WAX/GCB alone. When using the stacked cartridge, dGCB was not used.
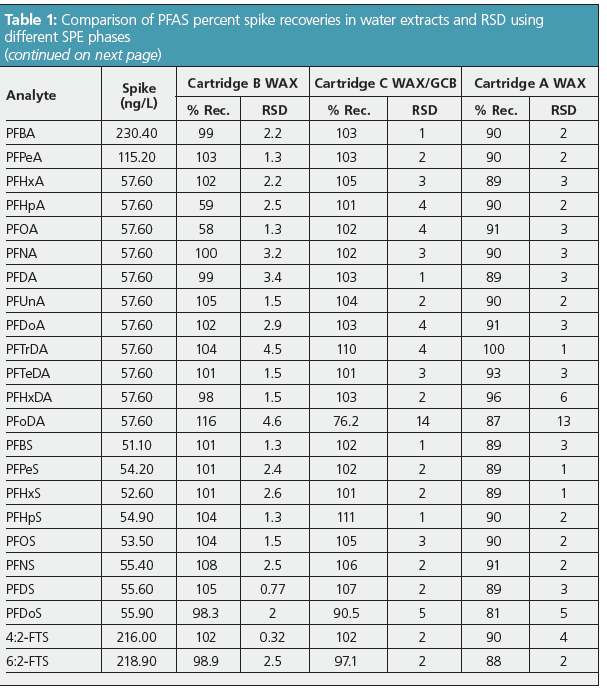
Figure 2 shows the extraction precision of the PFAS analytes from the four water samples as %RSD. These results were compared with those obtained from the single lab draft method. Overall, the precision was lower in this study compared with the published method. Though there were minor variations between the phases, the precision difference between the phases was not statistically significant within the sample size. Perfluorooctadecanoic acid (PFODA) was one of the standards tested per the California water board recommendations. This analyte had the poorest precision but was still well below 15%.
Comparison of Soil Extracts
Table 2 shows the comparison study results between the dGCB + WAX cartridge versus the single GCB/WAX cartridge of PFAS compounds recovered from spiked Ottawa sand extracts.
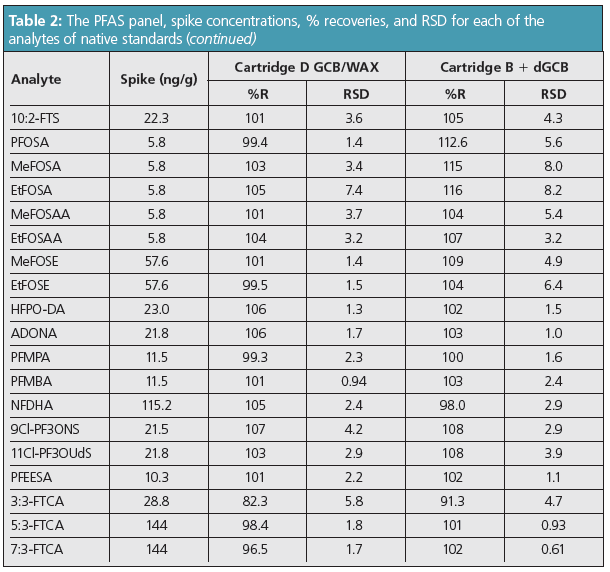
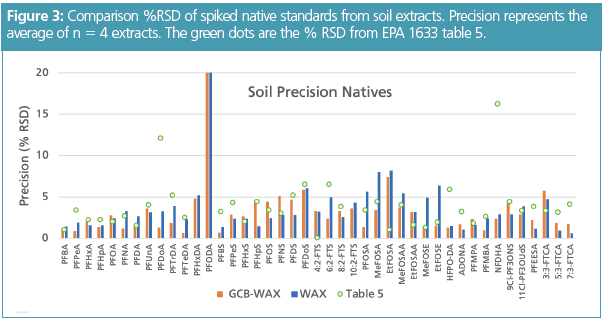
Per EPA 1633, soil extraction requires an initial 0.3% methanolic ammonium hydroxide extraction (see section 11.3.4– 11.3.7) (2), dGCB cleanup, and then the extract is passed over the SPE cartridge. Thus, the cleanup and SPE are in reverse order compared with the water samples. To simplify cleanup and SPE to a single step, a stacked cartridge that had dGCB on top of the WAX SPE was used. Figure 3 shows the extraction precision of the PFAS analytes from the soil extracts as %RSD using Ottawa sand as a reference material. These results were compared with those obtained from the single lab draft method. The %RSD for the native standards had excellent precision below 10% for all PFAS standards except for PFODA. Both procedures produced equivalent results for the cartridge comparison tests.
Conclusions
Whether using single WAX + dGCB or dual stacked WAX/GCB cartridges, these results demonstrated equivalence for an EPA 1633 PFAS panel from water and soil samples. Dual layer cartridges with the elimination of dispersive SPE provided equivalent performance to the WAX cartridges specified in Draft Method EPA 1633 for all 40 EPA 1633 parameters plus PFHxDA, PFODA, and 10:2FTS for both soil and water, as per IDOC requirements (section 9.1.2) and DOD QSM 5.4 table B-24. For the soil samples, only PFODA—which is not included in the EPA 1633 PFAS panel—failed the initial demonstration of capability (IDC) for both procedures. In our laboratory, the elimination of dispersive SPE reduced labour for each analytical batch (20 samples) by approximately 30 min for manual cartridge SPE cleanup. Elimination of the filtration step would provide a further 30 min labour reduction. Incorporation of the dual layer cartridges into the workflow enabled automation of the full cleanup procedure, with the potential for a significant reduction in labour and improvement in data reproducibility.
References
- https://www.epa.gov/sdwa/drinking-water-health-advisories-pfoa-and-pfos
- US EPA Draft Method 1633, “Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/ MS”: https://www.epa.gov/cwa-methods/cwa-analytical-methods-and-polyfluorinated-alkyl-substances-pfas#draft-method-1633
- https://www.epa.gov/system/files/ documents/2021-09/method_1633_draft_aug- 2021.pdf
- Phenomenex, Comparison of PFAS Recoveries Between Cartridge Format WAX/GCB vs. Dispersive GCB for DoD Compliance (TN- 0145): https://www.phenomenex.com/ documents/2022/05/21/00/09/comparison-of-pfas-recoveries-between-cartridge-format-waxgcb-vs-dispersive-gcb-for-dod-compliance-t
- EPA Draft Method 1633 for PFAS Analysis: Evaluation of SPE Extraction Options. NEMC Environmental Symposium 2022: www.nemc.us
- WATER CODE SECTIONS 13267 AND 13383 ORDER FOR THE DETERMINATION OF THE PRESENCE OF PER- AND POLYFLUOROALKYL SUBSTANCES AT PUBLICLY OWNED TREATMENT WORKSORDER WQ 2020-0015-DWQ: www. waterboards.ca.gov
Richard F. Jack is Global Market Development Manager for the environmental and food markets at Phenomenex Corporation. He has over 18 years’ experience with chromatography and mass spectrometry for the environmental, semiconductor, chemical, and pharmaceutical industries. Richard collaborates with global regulatory agencies to develop validated methods through new applications, instrumentation, column chemistries, and software. Richard is a former EPA Scientific Advisor for the EPA’s panel on hydraulic fracturing, a coauthor for EPA 557 and 557.1 along with ASTM D8001 and updates to D4327 and D6919 methods. He is currently the Second Vice Chairman for the ASTM D19 subcommittee on water analysis. He was previously a vertical marketing manager with Thermo Fisher Scientific, Dionex, and Hitachi High Technologies, where he designed analytical instrumentation, including ion chromatography (IC) and high performance liquid chromatography (HPLC) systems, pumps, autosamplers, and a variety of detectors. Richard received his Ph.D. in biochemistry and anaerobic microbiology from Virginia Tech University in Blacksburg, Virginia, USA. He received his master’s in ecology from the University of Tennessee in Knoxville, Tennessee, USA.
Sam Lodge is Business Development Manager - Environmental with Phenomenex in Torrance, California, USA.
Adam Robinson is the laboratory supervisor for LC–MS/MS analyses at Bureau Veritas’ Mississauga (Ontario) facility. In addition to maintaining operational excellence, he has contributed to the development of new analytical testing methodologies in support of the environmental and food science lines of business, including the new EPA Draft Method 1633.
Thushara Johnson is a laboratory technician at Bureau Veritas, Mississauga, Ontario. In addition to performing routine organic processing of environmental samples, she is a member of the PFAS development team; aiding BV and its clients with the unique analytical challenges and testing requirements of PFAS.
Adnan Khan is an analyst at Bureau Veritas, Mississauga, Ontario. Before joining BV, he was a research assistant at Western University in Canada where he published peer-reviewed articles. He is now applying that experience in his current role in the BV Research and Development team. He looks forward to further contributing in the R&D field of PFAS analytical chemistry.
Heather Lord joined Bureau Veritas in 2012 and is currently Senior Manager for Bureau Veritas’ Advanced Organic Testing Services. She manages all aspects of ultra-trace testing and reporting of priority and emerging organic pollutants such as PFAS, PCB, dioxin, furan, and PAH in food and environmental media.

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)















