The Integration of Microextraction Packed Sorbent (MEPS) into Multidimensional Strategies
The Application Notebook
LC/GC approaches to analysis are attractive because they combine the selectivity of solid-phase sorbents in the first dimension with the separating power and peak capacity of capillary GC in the following dimensions.
Peter Dawes, Ern Dawes, Roy Hibbert and Paul Wynne, SGE Analytical Science, Ringwood, Victoria, Australia.
Introduction
LC/GC approaches to analysis are attractive because they combine the selectivity of solid-phase sorbents in the first dimension with the separating power and peak capacity of capillary GC in the following dimensions. Their widespread use is limited because of the difficulty in desolvating the stream from the LC dimension without the solvent vapour passing down the GC column in significant quantity. An alternative approach to elution chromatography is to harness the specificity of the solid-phase process for digital chromatography using discontinuous changes in solvent polarity.
MEPS is a miniaturized SPE device with a typical void volume of less than 10 μL. This allows for injection directly into a GC with a large volume injector or for subsampling into a conventional split/splitless injector. This combined with its compatibility with autosampler syringes makes the MEPS format ideal for a digital LC–elution GC approach to analysis. In this application, we use the selectivity of an argentation sorbent to speciate a mixture of fatty acid methyl esters (FAME) on the basis of unsaturation in the first dimension and then to separate groups by non-polar GC–MS in the second dimension.
Experimental Detail
A SCX MEPS cartridge (propylsulphonate modified silica, SGE Analytical Science) was conditioned with 200 μL of 8% w/v silver nitrate in acetonitrile–water (10:1), then washed sequentially with acetonitrile (200 μL), acetone (200 μL), dichloromethane (200 μL).
A 37 FAME standard and a PUFA FAME standard (Supelco Inc., Bellefonte, Pennsylvania, USA) were mixed 1:1 in dichloromethane and 50 μL of this sample was drawn through the sorbent, then expelled at a flow-rate of 10 μL/sec. The sorbent was washed with dichloromethane–hexane (2 × 100 μL) and then eluted sequentially with dichloromethane (2 × 50 μL), acetone (2 × 50 μL) and acetone–acetonitrile (94:6, 2 × 50 μL).
GC–MS was performed on the eluted fractions using a 6890GC-5973N MSD (Agilent Technologies, Palo Alto, California, USA) and BPX5 column (30 m × 0.25 mm i.d., 0.25 μm film thickness, SGE Analytical Science). Injections of 1 μL were fast and splitless at 250 °C with a purge flow of 50 mL/min and a nominal inlet pressure of 0.7 psi. The oven temperature was initially 40 °C, ramped at 20 °C/min to 350 °C, then held for 2 minutes. The carrier gas was helium at a flow-rate of 0.5 mL/min in constant flow mode. The method used here in a decoupled mode is also suitable for coupled analysis from the MEPS cartridge directly into a PTV injector.
Results and Discussion
By necessity, argentation media (e.g., propyl-SO3–Ag+ ) have a high surface polarity generated by the ionic double layer of ion exchanger and silver cations. Such surfaces are wettable by aqueous samples, but extraction of essentially non-polar analytes from organic solvents may be rate limited and the sorbent capacity lower than is observed for other solid-phase mechanisms. However, the selectivity of the sorbent towards unsaturation in combination with its very low van der Waals capacity means that it can be used to speciate compounds of interest on the basis of degree of unsaturation. These normal phase characteristics make argentation a useful digital chromatographic technique because it uses solvents compatible with GC inlets. It is of particular use for the speciation of FAME mixtures in which unsaturated target analytes may be swamped by more abundant saturated FAME. In MEPS format, it allows the separation of FAME with two or more double bonds from saturated and monoenic analogues (Figure 1). Saturated and monoenic FAME was recovered without retention, dienic and trienic were eluted with some more unsaturated FAME. Finally the polyenic FAME was completely eluted by the inclusion of 6% acetonitrile to fully disrupt the silver ion-ene complexes.
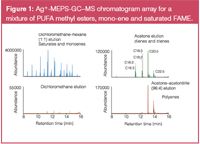
Figure 1
Conclusion
An Ag+ -MEPS-GC–MS method has been developed for the analysis of complex FAME samples. The technique generates a three dimensional array of chromatographic data by sequential analysis of discrete MEPS fractions. The incorporation of sample preparation into the chromatographic method not only allows for a greater degree of automation in sample processing but also provides additional separation capabilities.

SGE Analytical Science
7 Argent Place, Ringwood, Victoria 3134, Australia
tel. +61 3 9837 4200 fax +61 3 9874 5672
E-mail: support@sge.com
Website: www.sge.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



