Alternate Selectivity for Polar Compounds in Hydrophilic Interaction Liquid Chromatography (HILIC) Using a New Amino Type HILIC Column
The Application Notebook
Hydrophilic interaction liquid chromatography (HILIC) offers unique advantages for the separation of very polar compounds when compared to reversed-phase chromatography. A new silica based HILIC phase was developed to provide additional selectivity options in HILIC separations. The separation of water soluble vitamins on the new TSKgel NH2-100 HILIC column and on the well known TSKgel Amide-80 HILIC column demonstrates the differences in selectivity.
Regina Roemling,1 M. Sakata,2 Y. Kawai,2 H. Yamasaki2 and H. Moriyama,2 1Tosoh Bioscience, Stuttgart, Germany, 2 Tosoh Corporation, Tokyo, Japan.
Introduction
HILIC is used primarily to separate polar and hydrophilic compounds.1,2 Target applications for HILIC include the analysis of saccharides, glycosides, oligosaccharides, peptides and hydrophilic drugs. Altering selectivity plays a major role in maximizing resolution. The availability of an additional TSK-GEL HILIC phase provides a powerful tool for rapid method development.
Hydrophilic interaction chromatography
HILIC has similarities to normal phase chromatography with regard to the nature of the stationary phase. However, the eluents used for HILIC are similar to those known from reversed-phase chromatography. Typical mobile phases are mixtures of acetonitrile and water or aqueous buffers, applied in isocratic or gradient mode. It is commonly believed that in HILIC the aqueous content of the mobile phase creates a water rich layer on the surface of the stationary phase. This allows for partitioning of solutes between the more organic mobile phase and the aqueous layer. Hydrogen bonding and dipole–dipole interactions are the dominating retention mechanisms in HILIC mode. The number of polar groups, as well as the conformation and solubility of the sample in the mobile phase determine the elution order.
HILIC phases
Typical HILIC stationary phases are silica or polymer particles carrying polar functional groups (e.g., amino, amide or zwitterionic groups). TSKgel Amide-80 HILIC columns are well established for the analysis of glycans by HPLC or HPLC–MS.3,4,5 Packed with spherical silica particles that are covalently bonded with non-ionic carbamoyl groups they offer expanded stability. Conventional amino type HILIC columns have limited stability in aqueous solutions but sometimes the selectivity of an amino ligand might better suit the target HILIC application. Therefore, we developed a new HILIC phase, which combines the amino ligand functionality with high durability.
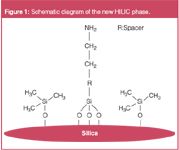
The new amino type HILIC phase is based on a 3 μm silica particle with 100 Å pores, which is treated with a special endcapping procedure. Amino groups are introduced step wisely after endcapping (Figure 1). The amino groups act as HILIC functional groups without any peak splits. Because of a high ligand density and large surface area TSKgel NH2-100 3 μm columns show the strongest retention for very polar compounds among the commercially available HILIC columns.

Figure 2 shows a comparison of the H-u plots of the TSKgel Amide-80 and NH2-100 HILIC phases. The optimum HETP for the amino type column is reached at a flow-rate of 1.2 mL/min at a pressure of 6 MPa. At increased flow-rates the H-u-curve is relatively flat. This allows using this column for fast separations at elevated flow-rates without impairing separation efficiency.
The availability of two TSK-GEL HILIC phases offering different selectivity features allows for adopting both, the stationary as well as the mobile phase to optimize the separation of a given sample. Available in a particle size of 3 μm, both columns are ideally suited for high efficiency HPLC as well as HPLC–MS analysis
HILIC Analysis of Water Soluble Vitamins
HILIC separations are performed either in isocratic mode with a high percentage of organic solvent or with gradients starting with high percentage of organic solvent and ending with a high portion of aqueous solvent, which is opposite to reversed phase. The elution order of compounds is usually inversed as well. As a result in HILIC mode polar compounds are very well separated according to increased polarity. We present the analysis of water soluble vitamins (Nicotinamide, Nicotinic acid, Pyridoxine and Vitamins B1, B2, B12 and C) as an example for the separation of polar compounds on the two available TSK-GEL HILIC phases. For difficult separations of the very polar vitamins of the B complex HILIC separation is advantageous over reversed-phase analysis, especially when combined with highly sensitive mass spectrometric detection.
Material and Methods:
Columns: TSKgel NH2-100 3 μm, 4.6 mm i.d. × 15 cm L TSKgel Amide-80 3 μm, 4.6 mm i.d. × 15 cm L
Mobile phase: 25 mM phosphate buffer (pH 2.5)/ACN = 30/70
Flow-rate: 1 mL/min
Temperature: 40 °C
Detection: UV @ 254 nm
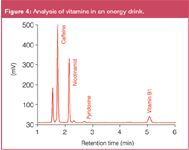
Sample: Figure 3: vitamin standard mixture Figure 4: energy drink (filtrated, diluted in ACN (1:1))
Injection: 5 μL
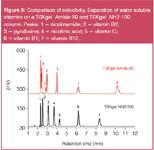
Figure 3 shows the separation of a standard solution of water soluble vitamins on a TSKgel NH2-100 column compared to the separation on a TSKgel Amide-80 column. Both columns have the same dimension (4.6 mm i.d. × 15 cm L) and particle size (3 μm). Flow-rate and mobile phase were identical as well. The elution order of the compounds varies when applying the same mobile phase to both columns: The new amino type column shows a stronger retention for nicotinic acid, vitamin C and vitamin B12 while retention of vitamin B1, B2 and pyridoxine is reduced. Figure 4 shows the analysis of a commercially available energy drink on the new amino-type HILIC column, after filtration and addition of the same volume of acetonitrile.
Conclusion
TSKgel Amide-80 HILIC columns have been used for years for a broad range of HILIC applications. The new 3 μm TSKgel NH2-100 columns provide an additional selectivity option, when increased retention or alternate selectivity is needed. This new bonded phase provides a powerful tool for robust method development in hydrophilic interaction liquid chromatography.
References
1. A. Alpert, J. Chromatogr., 499, 177–196 (1990).
2. Y. Iwasaki et al., J. Liquid Chromatogr. & Rel. Techn., 30(14), 2117–2126 (2007).
3. W. Laroy et al., Nature Protocols, 1, 397–405 (2006).
4. P. E. van der Steen et al., J. Biol. Chem., 281(27), 18626–18637 (2006).
5. H. Nakagawa et al., J. Chromatogr. B, 853(1–2), 133–137 (2007).

Tosoh Bioscience GmbH
Im Leuschnerpark 4
64347 Griesheim
tel. +49 6155-7043700
fax +49 6155-8357900
E-mail: Info.tbg@tosoh.com
Website: www.tosohbioscience.de

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.










