Application of Novel Ethylene Bridged Hybrid Particles for Hydrophilic-Interaction Chromatography
The Application Notebook
Using HILIC with highly efficient ethylene bridged hybrid (BEH) particles results in faster methods that exhibit improved polar retention, higher sensitivity, enhanced chromatographic resolution and significantly improved column lifetime.
Kenneth J. Fountain, Eric S. Grumbach, Jane Xu, Christopher J. Messina and Diane M. Diehl, Waters Corporation, Milford, Massachusetts, USA.
Introduction
Hydrophilic-interaction chromatography (HILIC) is a chromatographic technique that improves the retention of very polar species that are poorly retained in reversed-phase HPLC (RP-HPLC) by using a high organic, low aqueous mobile phase in combination with a polar stationary phase.
HILIC offers several benefits over RP-HPLC with regards to MS response and simplification of sample preparation methods.
Experimental
Separations were performed on a Waters ACQUITY UPLC system. Detection was performed with an ACQUITY TQD mass spectrometer in SIR mode (m/z 146.2 for acetylcholine and m/z 103.9 for choline).

Figure 1
Results
Comparison of ESI+ MS response in HILIC-and RP-UPLC for (1) acetylcholine and (2) choline is shown in Figure 1. Direct injection of an SPE eluate (5% NH4OH in ACN) from an Oasis MCX μElution plate (PN 186001830BA) onto an XBridge HILIC column (2.1 × 100 mm, 3.5 μm, PN 186004433) is shown in Figure 2.
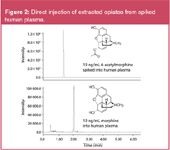
Figure 2
Conclusions
Novel BEH particles were found to be useful for HILIC separations and exhibit chemical resistance superior to that of silica particles at moderate pH over the course of 2000 injections. HILIC offers complementary selectivity to RP and was found to yield up to 10-fold improvement in ESI-MS response when compared to RP-HPLC. Separations are directly transferable between XBridge HILIC and ACQUITY UPLC BEH HILIC columns.
For the complete UPLC application note, visit www.waters.com/27112
© 2008 Waters Corporation. Waters, The Science of What's Possible, UPLC, ACQUITY UPLC, ACQUITY, XBridge and Oasis are trademarks of Waters Corporation.

Waters Corporation
34 Maple Street, Milford, Massachusetts 01757, USA
tel. +1 508 478 2000 fax +1 508 478 1990
Website: www.waters.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.










