Instrument Considerations in the Transfer of Chromatographic Methods, Part II: System Considerations
LCGC North America
Scientists executing a method transfer often do not have access to the originating system. Thus, alternative approaches to matching chromatographic results must be considered.
The process of transferring chromatographic methods between users and laboratories is often complicated and time consuming. This is the second installment of a three-part consideration of this common activity. In part 1, the focus was on the method itself. In this second part, the techniques and concerns about characterizing the systems in use in the originating laboratory and the new facility will be described. Given that the scientists executing the transfer often do not have free access to the originating system, alternative approaches to matching chromatographic results will be considered.
Transferring chromatographic methods among users and laboratories is a common and important activity that has often proven more difficult than expected. This is the second installment of a three-part discussion focused on the most rigorous method transfer leading to the duplication of results of the established methods. The first installment (1) described the aspects of the method that specifically affect the transfer of the method. In the future, the final part of this series will consider the details of aligning individual instrument modules. In this part, we address the chromatographic systems and how they may be characterized and compared for use in the transfer. It is generally assumed that modern instrumentation delivers the volumes, temperatures, and so on that are programmed in the control software. That is generally true for well-maintained systems. There are, however, subtle differences in the exact delivery and conditioning of flow that can have significant effects on the chromatographic results. Such details occur in all the system modules. We discuss here the characterization of the systems to identify these operational differences.
General Considerations
The chromatographic instrument itself is often the largest contributor to inconsistencies in the transfer of methods. The common principle applied for all other considerations, "Use exactly what was used in the originator's laboratory," is desirable here. It is, however, very often impossible to maintain that consistency. The instruments used in various laboratories are often different models or brands, and it is not usually financially sensible to purchase chromatography instruments for each specific new method to be implemented. Furthermore, the usable lifetime of a method is often much longer than that of an instrument. Duplicating instruments, therefore, may not be possible to begin and execute a method transfer. We must, then, consider the differences among instruments that can affect method transfer. The transfer of a method from one instrument to another may require some adjustment of the method. Many laboratories adhere to the guidelines found in Chapter 621 of the current United States Pharmacopeia. The currently applicable chapter specifically states:
Adjustments to the specified chromatographic system may be necessary in order to meet system suitability requirements. Adjustments to chromatographic systems performed in order to comply with system suitability requirements are not to be made in order to compensate for column failure or system malfunctions. Adjustments are permitted only when . . . adjustments or column change yields a chromatogram that meets all the system suitability requirements specified in the official procedure (2).
These guidelines, often mentioned as "<621>", specify changes to the method that may be implemented without revalidating the method. The chapter has been summarized in many places, but the original document should always be consulted. We will allude to specific items in these guidelines in the context of specific challenges in method transfer. It should be emphasized that many laboratories follow these limits and practices, but they are not universal regulations. They are absolute requirements only for the compendial methods of the USP.
The Fluid Path
There are important factors associated with the fluid path in general; the pumping or solvent delivery system; the sample introduction or injector system, and the detector. Each component will be considered for its potential impact on the method in terms of altered retention time as it may affect peak identification and resolution; altered chromatographic selectivity as it may affect resolution and quantification; and peak shape as it may affect resolution and sensitivity.
The fluid path of the instrument includes all the tubing, and other elements where liquid moves through the system. To consider the impact on transfer, we must distinguish between segments that transport the sample and segments that are only exposed to the mobile phase. We generally assume that the fluid is unaltered during this transport, but this may not be absolutely true. While the instrument may create trace chemical changes in the mobile phase, such as metal leaching, this seldom complicates method transfer, since materials of construction are consistent across models and manufacturers. The worrisome effects are physical. In addition, the tubing dimensions create some timing differences.
The time to transit the fluid path is often of great concern to laboratory scientists using chromatographs and transferring methods; these scientists frequently comment that tubing must be shortened and modules placed closer together. In fact, such changes are almost imperceptible. As a point of reference, a 3 m piece of 0.005 inch (~127 µm) tubing has a volume of about 12.7 µL. So the transit time at 1.00 mL/min is only 0.01 min or 0.75 s. Even doubling the tubing diameter only increases the time to 3 sec. Larger differences are typically allowed for flow rate or retention time variability in typical system suitability specifications. So, there is little reason to focus on tubing length as a factor in time offset.
The tubing in the system also contributes some mixing or dispersion, primarily because of laminar flow differences between the tubing wall and the center. The magnitude of this effect is dependent on both the diameter and length of the tubing. It is important here to distinguish between parts of the flow path used for mobile-phase transport only and the parts downstream from the injector where the sample is in the flow path. As a general rule, the upstream parts of the system can contribute some mixing to blend the mobile phase. However, as discussed above, the volumes and the residence time are so small that there is no significant contribution to mixing. There is, of course, substantial solvent blending in all systems, and we will discuss that below in the context of the pumping system.
Dispersion in the fluid path downstream from the injector has an impact on both peak shape and resolution. This has been considered in detail in other investigations (3). The largest contribution to dispersion during sample transport originates with the tubing diameter, with length as a smaller contributor. It is, therefore, important during method transfer to ensure that the tubing used for connecting the modules of the target system are the smallest possible diameter and the shortest length. In following this guideline, however, it is not necessary to take extreme measures. Consider the expected volume of the peaks as they elute from the column. Many standard methods have peak volumes near or above 50 µL. The effect of a 10 µL tubing volume will be relatively small. Even for the most modern ultra-high performance liquid chromatography (UHPLC) methods, with peak volumes near 10 µL, 0.004 inch (~102 µm) contributes about 2 µL per foot, and 0.0025 inch (63.5 µm) is less than 1 µL per foot.
When implementing the above tubing considerations, many scientists overlook the back pressure that can arise simply from flow through tubing. At the midpoint of a water–methanol gradient, at 1 mL/min, the resistance to flow through 1 m of a 0.0025 inch (~63.5 µm) tubing generates a back pressure greater than 5000 psi. For this reason, the tubing used to assemble a system should be no smaller than required for minimizing dispersion.
Pumps
The pumping, or solvent delivery system, has received the most attention of any instrument module in terms of its effect on method transfer. Isocratic separations are reasonably simple because modern systems reliably deliver the specified flow rate from a reservoir of preblended solvent, with the preparation constraints discussed in part I of the series. Gradients are much more complicated to duplicate between systems. We assume that the pump is delivering liquid flow at the programmed rate, and that the percentage composition accurately corresponds to the programmed value. We also expect that any gradient follows the intended profile and that the specified composition reaches the column at the intended time. All modern chromatographic systems closely approach these assumptions, but there are always deviations from the ideal. The differences in the deviations between different brands or models of pumps create complications for method transfer. The differences can affect retention time and selectivity. It is less well recognized that the differences can also affect column regeneration and reequilibration. Most scientists consider the system volume to be the major source of the deviations so method transfer strategies are often based on equalizing the system volume differences.
System Volume
The system volume, also called dwell volume, void volume, or delay volume, is the amount of liquid from the point where two, or more, solvents meet and the point where the blended solvents reach the head of the column. Two systems with different dwell volumes, delivering the same gradient, will give different retention times. It has long been recognized that characterizing the volume of a system is the key first step for maintaining constant retention time in a method transfer. There are many published and freely circulated procedures for measuring system dwell volume (4,5). The general approach is to prepare two batches of the same solvent, one of which is spiked with a UV-absorbing marker, such as uracil, acetone, propyl paraben, or caffeine. The column is replaced with a short length of small inner diameter (i.d.) tubing. A gradient is run from 100% A to 100% B over 20 min, followed by a return to 100% A over 5 min. A hold, typically 5 min, is incorporated at 100% B, and at the return to 100% A. The gradient is observed as the UV absorbance trace at the wavelength appropriate for the selected marker. The volume of the system is best measured at the midpoint of the gradient. The difference in time between the programmed midpoint and the observed midpoint is multiplied by the flow rate to obtain the system dwell volume. Although it is sometimes suggested that the volume be measured with an instantaneous step from A to B or, alternatively, at the first liftoff of the gradient trace, the midpoint is much preferred as reflecting a steady state transfer through the mixing volume of the system, as discussed below. Although system volume values typically are published by instrument manufacturers, these values are seldom ideal for method transfer experiments because the measurement procedures are not consistent from one manufacturer to another. It is, therefore, necessary to measure the particular systems in use. For most purposes, the best method is the one recommended by the manufacturer of the system, but the overriding consideration is that the same method be used to measure the volume of both the original and the target system. A typical useful procedure is shown in Figure 1 (5).
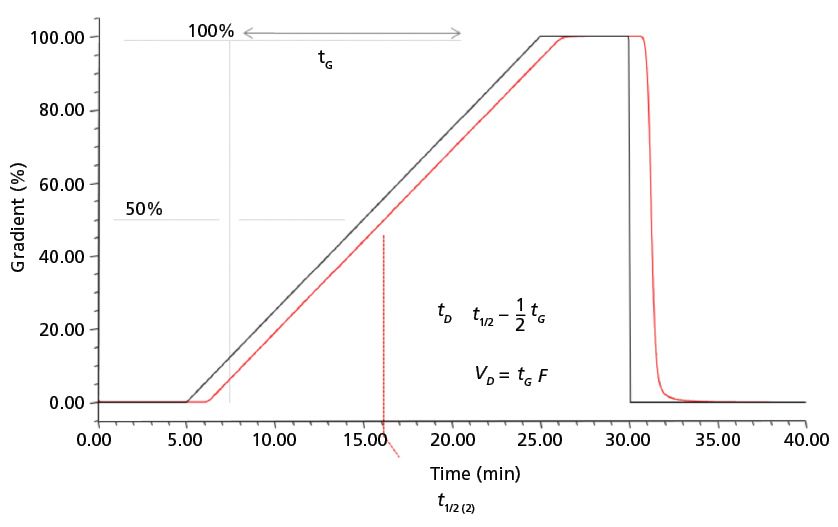
Figure 1: The measurement of the volume of a chromatographic system is based on running a gradient after removing the column. Mobile-phase A = water. Mobile-phase B = water with 10 mg/mL caffeine. Detection wavelength = 273 nm. The strong solvent is spiked with a UV absorbing marker (in this case, caffeine). Mobile-phase gradient: hold at 0% B for 5 min, then 0-100% B in 20 min, 100% B for 5 min, 100-0% B in 5 min; flow rate: 1 mL/min. (Reprinted with permission from reference 2).
The measurement of dwell volume does not ensure perfect replication of the gradient to be transferred. When the gradient trace generated during the measurement is examined in detail, it is always observed that the offset time or volume is not constant throughout the gradient profile. This variation is a consequence of several factors. Systems generally create the programmed percentages by blending volumes of each solvent. There is, however, some inaccuracy associated with the nonadditive effects associated with pairs of solvents. In addition, all instruments make some adjustment for compressibility changes. The much larger source of changes in the gradient profile is the mixing that is incorporated in all systems. Gradient systems must include a volume for mixing of the proportioned solvents. Many different designs have been used for mixers, but they all must meet the two criteria of stable, reproducible retention times and ripple-free baselines. The ripples reflect solvent concentration inhomogeneities that may alter retention and will certainly complicate quantitative integration of the peaks. The alternative mixer designs all are intended to smooth these ripples by making the solvent changes uniform with time. To achieve this, the mixer must have a common volume that is large enough to span the period of the ripple. This common volume, however, also distorts the programmed profile of the gradient. When a change in composition is initiated, that change is intended to be uniformly distributed throughout the mixer volume. But that change, now much smaller because it is diluted by the mixer volume, begins to emerge from the mixer immediately. In other words, it reaches the head of the column in much less than the physical volume of the system. Over the course of the gradient, this phenomenon becomes less significant as the series of small composition increments settles into a steady state change. This is the basis for recommending that the system volume be measured at the gradient midpoint. Measurement at the start of the rise in the gradient monitoring trace gives a much smaller volume than the physical volume, or the steady state condition.
Mixers
The differences among mixers can have three effects on chromatography. First, with a smaller system volume, the leading edge of the gradient reaches the column and causes peaks that elute very early in the gradient to be shifted to an earlier retention time. Second, because the entire mixer volume must be flushed with the final conditions of the gradient before that strong solvent is delivered to the column, the column may not be regenerated on one system as compared to the other. This can lead to a drift in retention times over a series of injections. Third, on return to initial, the entire mixer volume must again be flushed before re-equilibration can begin. Again, the two systems may differ in the equilibration to initial conditions for the next injection. In this case, the two systems may prove reproducible but different from one another. These three problems have different symptoms, and solutions, from a simple mismatch in measurement of dwell volume. Strategies for correcting system volume differences will be discussed in part III.
Gradient Mixing
There is one additional difference among chromatographic systems that must be considered. Pumps are usually classified as multi-pump gradient, high-pressure mixing systems or as single-pump gradient, low-pressure mixing systems. The high-pressure mixing systems create specific compositions by varying the flow from two or more pumps to generate the desired solvent percentages. The low-pressure mixing approach uses a solenoid valve where each port opens for a percentage of the valve cycle time corresponding to the programmed composition. This series of solvent aliquots is carried to the pump through a single tubing piece. Some blending of the solvent segments occurs in the transport tubing and then in the pump heads as the mobile phase is brought to the pressure and flow for the method. Both system designs can work very well in terms of compositional accuracy and precision. The system volume is usually somewhat smaller with a multi-pump gradient system, whereas the single pump system can provide more convenience as described in part I in the context of solvent preparation.
There is an additional characteristic associated with low-pressure mixing systems that is not generally recognized. The fluid in the transfer tubing from the proportioning valve enters the pump head as a block corresponding to the delivery volume of the pump head. That series of solvent segments is mixed, more or less completely, into a single packet at the composition specified at that time. That blended volume of solvent is then delivered into the flow path towards the column inlet. Rather than the smoothly blended continuous gradient that we envision, the pump transmits a series of small steps that are somewhat smoothed at the edges of the packets. This flow and composition pattern is further complicated by a mechanical characteristic of pumps. When the piston, or plunger, expels the liquid from the head, it does not dispense all the liquid, because there is space between the surface of the piston and the walls of the pump head. This unswept liquid can be a substantial fraction of the total volume of the pump head, on the order of 40% of its physical volume. That residual volume mixes with the incoming mobile phase of the next segment of solvent aliquots. The result of this sequence of events is that the mobile phase is diluted with the composition of the previous pump cycle, which was also similarly diluted. This contributes to the larger system volume associated with single-pump systems, and, more importantly, adds to the required time to reach the regeneration step in a gradient method and also to complete the return to initial conditions for re-equilibrating for the next injection.
Tools for Instrument Calibration in Method Transfer
The physical factors that directly affect the shape of the gradient in a given system have elicited interest in alternatives to the common and simple ways to estimate and use the system volume. One interesting alternative is the use of marker compounds to calibrate the system. A more general tool, called "Measure Your Gradient," has been developed to evaluate the shape of the gradient on various systems (6). This tool can be implemented from an open-source web tool: http://www.measureyourgradient.org/index.php. For this approach, the intent is to recreate the identical gradient on the two systems in question. The gradient table entries will be different, but the solvent composition delivered to the column will be identical. The "Measure Your Gradient" tool uses a very exactly defined mixture of test compounds along with a specified column, mobile phase, and method. This test is run on both systems and the retention times are entered into the software. The gradient is calculated to give a specific array of retention times. Because this approach provides a multi-point calibration that addresses the several physical factors discussed above that make gradient transfer imperfect, it provides a very good adjustment for method transfer. It is, however, limited if the information is not available about both systems in the method transfer. This problem arises when implementing methods that are even a few years old where the systems may no longer exist. Even in the case where current instrument systems were used to develop the method, those instruments may not be available to the laboratory responsible for the transfer. This same obstacle can interfere with the conventional measurement strategies discussed above. When the observed system volume is to be used to match the gradients delivered by two systems, the same measurement protocol must be used on both systems. That may simply not be possible.
An alternative to measuring the exact system volume can be suggested for transfer of the separation method. The originator method (the method being duplicated), should provide system suitability criteria specifying the retention time for each sample component that is to be measured. Use the established method on the new system. This initial experiment may not meet the specifications, but the major analyte peak will be recognizable. Compare the retention time of this peak on the new system with the time in the specifications. The difference in retention time multiplied by the flow rate gives the difference in system volume between the two systems. This trial can then be repeated after applying the strategies discussed in part III to match the volumes between the two systems. After a few iterations of this empirical adjustment, the chromatogram should be close to meeting specification. If there are still discrepancies, apply a correction to the target system that minimizes all the differences in time.
Conclusions
We have considered the contribution of system characteristics to transferring chromatographic methods. The possible differences between the originating and the target systems can alter resolution by changing the characteristics of solvent delivery and by altering dispersion of the separated peaks as they migrate through the system. Consideration must also be given to the resistance to flow within the system tubing and the blending of solvents. In our final part of this three-part series, we will consider the modules that comprise each system and the ways that they can be aligned for consistent results.
References
(1) T.E. Wheat, LCGC North Am. 36(9), 693–696 (2018).
(2) General Chapter <621> "Chromatography" in United States Pharmacopeia 40 National Formulary 35 (USP 40-NF 35, United States Pharmacopeial Convention, Rockville, Maryland, 2017), pp. 508-520.
(3) F. Gritti, T. McDonald, and M. Gilar, J. Chrom. A 1420, 54-65 (2015).
(4) J. W. Dolan, LCGC Europe, 19(6), 336-343 (2006).
(5) P. Hong and P.R. McConville; Waters Corporation White Paper; 720005723EN; (c)2016 Waters Corporation (2016).
(6) M.H. Magee, J.C. Manulik, B.B. Barnes, D. Abate-Pella, J.T. Hewitt, P.G. Boswell; J. Chrom. A 1369, 73-82 (2014).
Thomas E. Wheat is a principal scientist with Chromatographic Consulting, LLC in Hopedale, Massachusetts. Direct correspondence to: chromatographic.consulting@gmail.com.

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.












