UCT - In-Well Hydrolysis Using UCT’s Refine Ultra-Filtration Technology
UCT’s Refine Ultra-Filtration Technology allows for sample precipitation and filtration to occur simultaneously in the individual column or well. A novel, hydrophobically treated, submeter frit combination facilitates the removal of sample proteins without a complicated extraction.
Procedure
Sample Extraction
a) Place a collection plate under the Refine Ultra-Filtration Plate
b) Add 250 μL of urine and 250 μL Abalonase Ultra Enzyme working solution.
c) Hydrolyze for 30 min at 55°.
d) Further dilute sample by adding 500 μL D.I. H2O.
e) Mix the sample. This can be executed via vortexing at maximum speed or multiple pipette aspirations and dispenses.
f) Filter the sample using one of the following techniques:
1. Centrifuge: For 5 min at 500 g or until filtrate is collected.
2. Vacuum: Apply vacuum at ~20” of Hg for up to 5 minor until filtrate is collected.
3. Positive Pressure: Apply 2–5 psi using positive pressure for up to 5 min or until filtrate is collected.
Instrumental
LC-MS/MS: AgilentTM 1200 HPLC and AB SciexTM 4000 Q Trap (MS/MS)
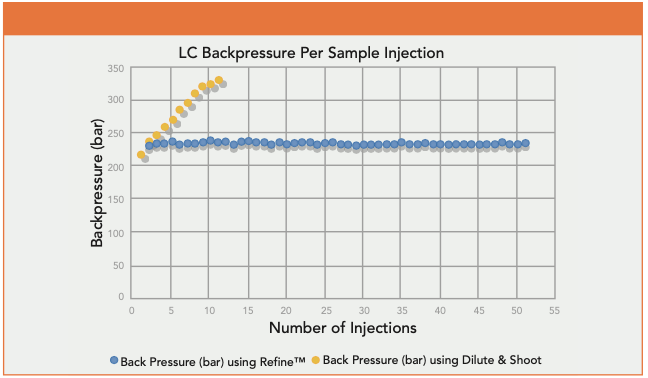
Figure 1: Backpressure per sample injection of Refine Ultra Filtration technology versus a dilute and shoot preparation for hydrolyzed urine samples.
Column: UCT Selectra® DA HPLC Column 100 × 2.1 mm, 3 μm
Guard Column: UCT Selectra DA Guard Column 10 × 2.1 mm, 3 μm
Injection Volume: 10 μL
Mobile Phase A: D.I. H2O + 0.1% Formic Acid
Mobile Phase B: MeOH + 0.1% Formic Acid 0.30 mL/min
Column Flow rate: 0.30 mL/min
Conclusion
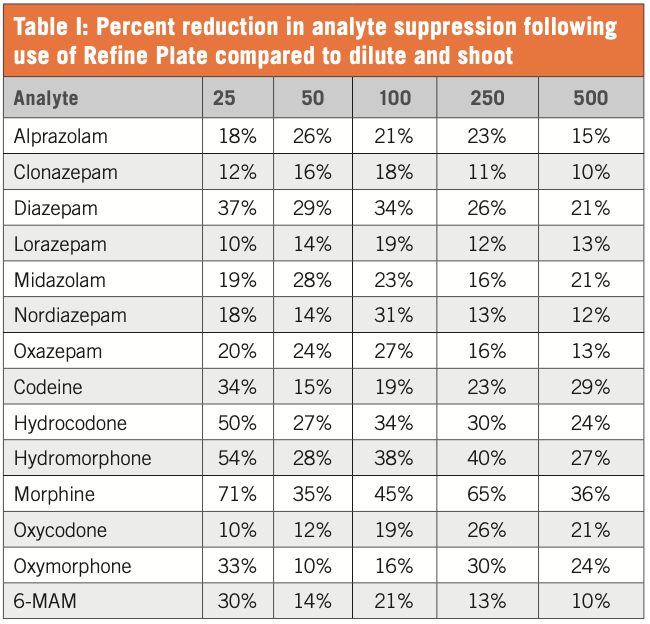
Using the above procedure, the Refine Ultra-Filtration technology allowed for a reduction in instrumental backpressure and removal of unwanted matrix components in urine samples. Overall, this allows for an end user to have enhanced analyte selectivity and an increase in HPLC/UPLC column life.

UCT, LLC
2731 Bartram Rd, Bristol, PA 19007
tel: (800) 385-3153
Website: www.unitedchem.com

A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.
The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.









