Shodex - Oligonucleotide Analysis Using a Polymer-Based Diol Column
Oligonucleotide therapeutics can treat genetic and metabolic disorders, and be used in vaccines and cancer treatments. They have the ability to specifically bind with deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), allowing control over specific gene expression linked to genetic diseases. The development and quality control of oligonucleotide therapeutics requires highly selective and sensitive analytical methods, including LC–MS measurements. The method most often used for the analysis of oligonucleotides has been reversed-phase liquid chromatography with the use of ion-pair reagents. Unfortunately, the ion-pair reagents tend to remain on the LC system and cause several other problems. Ion-exchange chromatography is another method commonly used, but it requires using highly concentrated salt in the eluent, which is not ideal for MS detection.
The ShodexTM HILICpakTM VN-50 2D column used in this application is a high-performance HILIC column, packed with multiporous polyvinyl alcohol polymers modified with diol functional groups. The 2.0-mm internal diameter of the column is designed to work well for LC–MS measurements. The VN-50 series has successfully measured various oligosaccharides. It was shown to be effective for oligonucleotide analysis. This column analyzed oligonucleotides with a gradient elution of a volatile basic solvent and acetonitrile.
The Shimadzu Nexera/LCMS-8030 Plus system was used with Shodex HILICpakTM VN-50 2D (2.0 mm I.D. × 150 mm; particle size 5 μm; pore size 100 Å). High-pressure isocratic or linear gradient was used with Eluent A (ammonium formate aqueous solution) and Eluent B (acetonitrile) were used. The flow rate was set at 0.2 mL/min and the column temperature was set at 40 °C. The two types of oligonucleotide therapeutics are categorized under DNA and RNA. In this application, an unpurified, salt-free (20 mer): 5’-ATACCGATTAAGCGAAGTTT-3’ DNA oligonucleotide was dissolved in ultrapure water.
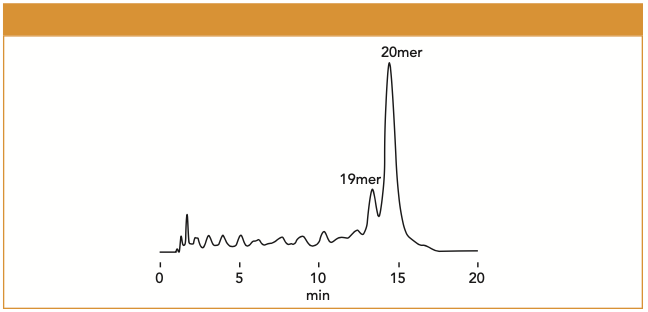
Several solvents were evaluated before choosing a suitable eluent including ammonium formate, ultrapure water, aqueous ammonium hydrogen carbonate, and ammonium water. The DNA oligonucleotide was only retained by ammonium formate, leading to its use as Eluent A. The sample was evaluated under different concentrations of Eluent A and different ratios of Eluent A/B for the gradient elution. When an isocratic elution of 100 mM ammonium formate with 57% acetonitrile was used, the main component of the sample, 20 mer DNA oligonucleotide, was detected around 15 min. MS data revealed that the DNA oligonucleotides smaller than 19 mer were detected before the 20 mer DNA. The smaller mers eluted in the order of smallest to the largest degree of polymerization. As the degree of polymerization increased, the hydrophilicity increased, therefore, the main separation mode worked in this analysis was assumed to be HILIC.
Smaller DNA oligonucletides were not sufficiently separated in this method. Gradient conditions were modified to improve separation. The analysis time can be shortened by decreasing the concentration of acetonitrile.
After comparing various options for the analysis of 5’-ATACCGATTA-AGCGAAGTTT-3’ DNA oligonucleotide, we found the ideal conditions to be (A) 50 mM HCOONH4 aq. (pH 9.5)/ (B) CH 3CN linear gradient; (B%) 64 to 58% (0 to 10 min), 58% (10 to 15 min), 58 to 64% (15 to 15.01 min), 64% (15.01 to 20 min) at 0.25 mL/min at 40 °C.
This application demonstrated the feasibility of analyzing up to about 30 mer oligonucleotides using the polymer-based diol HILIC column ShodexTM HILICpakTM VN-50 2D. The method is very selective by eluting the oligonucleotides by degree of polymerization. The gradient elution of 50 mM ammonium formate/acetonitrile used in this method was very simple, especially when compared to the commonly used reversed-phase method that requires an addition of ion-pair reagent or to the ion-exchange chromatography method that requires an addition of highly concentrated salts. Moreover, as the ammonium formate/acetonitrile eluent used here is volatile, it has an advantage in simplifying the desalting process during purification. The polymer-based packing material also allows the use of al- kaline washing solutions, which helps prevent the nonspecific adsorption and carryover related problems that are concerns in the analysis requiring high precision and accuracy, or in the preparative scale measurements.
Therefore, the methods using the VN-50 series columns introduced in this application have high potential in the development, quality control, and the purification of oligonucleotide therapeutics.
Figure 1: Analysis of oligonucleotides using Shodex HILICpak VN-50.

ShodexTM/Showa Denko America, Inc.
420 Lexington Avenue, Suite 2335A, New York, NY 10170
tel: (212) 370-0033, X 116; fax: (212) 370-4566
Website: www. shodexhplc.com










