Improving Injection Consistency Using N-Methyl-2-Pyrrolidone as a GC Syringe Wash
LCGC North America
Optimizing peak shape in capillary gas chromatography (GC) is essential for the consistent, accurate analysis of residual solvents in pharmaceutical compounds.
Optimizing peak shape in capillary gas chromatography (GC) is essential for the consistent, accurate analysis of residual solvents (that is, trace levels of organic volatile chemicals present from manufacturing) in pharmaceutical compounds. Band broadening can result in peaks that can exhibit increased tailing and greater variability in peak area (1,2). This, in turn, can affect analysis accuracy by loss of resolution between compounds and failing data acceptance criteria (for example, replicate percent relative standard deviation [%RSD]).

Various factors related to the instrument's set up have the potential to affect chromatography and peak area reproducibility. Slow detector responses can distort peak shapes (3,4). Peak shapes and peak maxima can be affected adversely by larger detector time constants, particularly for peaks with half-widths of 0.5 s or less. Also, low data sampling rates can reduce the reproducibility of the peak start, apex, and peak end, as well as the accuracy of a peak's shape (3).
Stationary phase partitioning, longitudinal diffusion, and resistance to mass transfer in the mobile phase are the classic processes that affect peak broadening inside the column. The effects of these processes are expressed in the Golay equation, which can be employed to optimize performance based upon the type of column and carrier gas linear velocity (5,6). The Golay equation is defined as H = B/u mean + C × u mean, where B is the longitudinal diffusion in the mobile phase, C is the resistance to mass transfer in the mobile and stationary phases, and u mean is the average carrier gas velocity. Stationary phase partitioning is of negligible contribution for columns with film thicknesses of 0.25 μm or less; however, above 0.5 μm, the partitioning effect can be significant enough to cause peak asymmetry (7). For any column, reversible solute adsorption to the stationary phase will be manifested as peak tailing (4). Also, analytes spread out to some extent as they diffuse through the mobile phase; however, all peaks should be affected equally by this gas-phase peak broadening (7,8).
Proper column installation is also important to reduce problems with reproducibility and peak tailing. Excessive dead volume at the column fittings and leaking connections in the carrier gas lines, instrument bulkhead, and the column ends are contributors to band broadening and lack of reproducible peak areas (4,9,10). In addition, peak tailing can be particularly pronounced due to the vaporization of certain solvents in the injector port, especially when using a packed injector port, as opposed to a capillary injector port, for capillary column applications (2,10).
If peak area reproducibility or peak tailing are still problematic after these factors have been eliminated or minimized, another factor to examine is the autosampler syringe. Unless all of the sample matrices being analyzed are very clean, nonvolatile residue and particulates eventually will build up within the barrel of the syringe, causing problems with injection reproducibility by degrading the fit of the plunger within the barrel (11). For this reason, most autosamplers are programmed to rinse the syringe with sample solvent between injections. However, more volatile solvents (for example, methanol and acetone) and certain sample matrices can increase the speed of residue accumulation even with rinsing between injections. The end result of slower or inconsistent injections is problematic reproducibility and issues with poor peak shape. Replacing or cleaning a syringe will solve the problem; however, replacing or cleaning a syringe several times during the course of sample analyses requires significant analyst time and effort.
Method redevelopment might be required for implementing alternative sample solvents to reduce residue accumulation in the syringe plunger, or for changing the instrument configuration (for example, changing from packed to capillary injection port, changing the type of column used, and so forth). Given the time and cost associated with method redevelopment, an alternative means of improving syringe performance is desirable.
In the case of the autosampler syringe, a simple way to address the issues of band broadening and reproducibility is to identify a material that can improve syringe lubrication, eliminating the need to replace syringes throughout a sample set. The ideal substance would be compatible with the analytical method in question; a liquid at room temperature; and sufficiently volatile to elute during the temperature program prescribed in the analytical method. For GC–FID residual solvents methods, one substance that is a viable candidate is N-methyl-2-pyrrolidone (Figure 1).
Figure 1
N-Methyl-2-pyrrolidone has been used in various environmental, petroleum, and pharmaceutical applications as a polar solvent for aromatic and C5+ aliphatic hydrocarbons, residual solvents, and parenteral pharmaceutical products (12–15). It is more viscous than water (1.65 cP) and has a dielectric constant of 32.2 at 25 °C (16). N-Methyl-2-pyrrolidone is well suited for residual solvents analysis with GC–FID because it is miscible with water and organic solvents, it can solubilize various pharmaceuticals, and it has a boiling point high enough (202 °C) to be eluted after most common residual solvents (14,15,17).
To explore the use of N-methyl-2-pyrrolidone as a viable syringe lubricant to improve result reproducibility and peak shape, three GC–FID residual solvent methods that had exhibited repeated issues with poor standard %RSD and peak tailing were examined.
Experimental
Instrumentation: Agilent 6850 and Agilent 6890 gas chromatographs (Santa Clara, California) with flame ionization detectors were used throughout these experiments. A packed or capillary injection port and an Agilent 10-μL autosampler syringe were used according to the individual analytical method. Syringe wash vials were set up for sample solvent in the wash 1 position and N-methyl-2-pyrrolidone in the wash 2 position. Data were collected using Waters GlobalChrom Data System (GCDS) and Empower software (Milford, Massachusetts). The GC conditions and temperature program for each method are listed in Table I.

Table I: GC conditions and temperature programs for the methods used in the study
Chemicals: N-Methyl-2-pyrrolidone (99.5% anhydrous) was purchased from Acros (Morris Plains, New Jersey). Purified water (Millipore, Billerica, Massachusetts, or equivalent), high performance liquid chromatography (HPLC)–GC grade acetone purchased from Burdick & Jackson (Muskegon, Michigan), and HPLC–GC grade methanol purchased from EMD (Gibbstown, New Jersey) were used as sample solvents. Acetone (99.9+% Chromasolv), methanol (99.8% anhydrous), 2-propanol (99.5% anhydrous), isoamyl acetate (98% reagent grade), N-amyl acetate (99%), isoamyl alcohol (99+%), and dimethyl sulfoxide (99.9+% ACS reagent grade) were all purchased from Sigma-Aldrich (St. Louis, Missouri). N-Amyl alcohol (99.2%) was also purchased from Acros. Reference standards of Lilly compounds pertaining to each method were obtained from Lilly Reference Standards Lab (Eli Lilly and Co., Indianapolis, Indiana).
GC Columns and Conditions
Columns and conditions used are listed in Table I.
Sample Preparation
All analyses used 1-μL injections.
Method 1: Samples were prepared in purified water at the following test concentrations: acetone, methanol, and 2-propanol each at 0.005 mg/mL; acetone, methanol, and 2-propanol each at 0.0005 mg/mL; and Lilly Compound A at 5 mg/mL.
Method 2: Samples were prepared in HPLC-grade acetone at the following test concentrations: isoamyl acetate, N-amyl acetate, isoamyl alcohol, and N-amyl alcohol each at 0.25 mg/mL; isoamyl acetate, N-amyl acetate, isoamyl alcohol, and N-amyl alcohol each at 0.00375 mg/mL; and Lilly Compound B at 35 mg/mL.
Method 3: Samples were prepared in HPLC-grade methanol at the following test concentrations: dimethyl sulfoxide at 0.025 mg/mL; and Lilly Compound C at 25 mg/mL.
Results and Discussion
The three GC–FID residual solvents methods examined often exhibited poor peak tailing and standard peak area (SPA)%RSD during normal execution. In practice, each of the methods required the use of a new syringe every time a new sample set (for example, system suitability solutions, standards, samples) was analyzed because the syringe plunger regularly seized after a few injections. Analysts spent considerable time and effort to ensure the methods performed appropriately; additionally, poor peak tailing and SPA %RSD often required repeat testing. Peak tailing for these methods is defined as w/2f, where w is the peak width at 10% height, and f is the time from peak start to peak maximum as measured at 10% peak height.
The experiments for each analytical method using N-methyl-2-pyrrolidone in a second syringe wash vial were compared with historical data that used only sample solvent as a syringe wash to assess method performance. Sample solvent blanks and quantitation limit samples were analyzed to obtain the elution profile for N-methyl-2-pyrrolidone and examine any potential interference from this compound. In the case of Method 3, the dimethyl sulfoxide standard was used in lieu of a reporting limit sample because its response is near the limit of quantitation. Standard solutions were analyzed to obtain values for peak tailing and SPA %RSD. Method 2 does not use peak tailing for a system suitability criterion; therefore, only SPA %RSD values were evaluated.
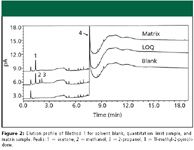
Figure 2
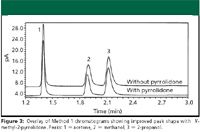
Figure 3
Method 1: The peak shape for methanol and 2-propanol are critical for accurate quantitation due to the close elution of the two peaks. Injection of the sample solvent blank and quantitation limit sample showed no interference of N-methyl-2-pyrrolidone with the peaks of interest (Figure 2). Peak shape for each analyte was improved (Figure 3), resulting in baseline separation of methanol and 2-propanol. Improved reproducibility for peak tailing and SPA %RSD were obtained when N-methyl-2-pyrrolidone was used as a syringe wash (Table II). Of note is the marked improvement in methanol SPA %RSD, which failed the acceptance criterion frequently before the use of N-methyl-2-pyrrolidone as a syringe wash.
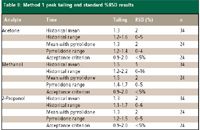
Table II: Method 1 peak tailing and standard %RSD results
Method 2: Injection of the sample solvent blank and quantitation limit sample showed no interference of N-methyl-2-pyrrolidone with the peaks of interest (Figure 4). Improved results for SPA %RSD were obtained when N-methyl-2-pyrrolidone was used as a syringe wash (Table III). Although historically, the method performed adequately in terms of reproducibility, SPA %RSD was improved approximately twofold by using N-methyl-2-pyrrolidone as a syringe wash.
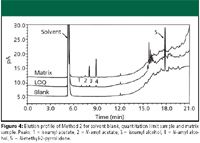
Figure 4
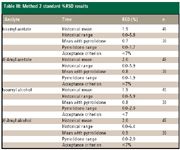
Table III: Method 2 standard %RSD results
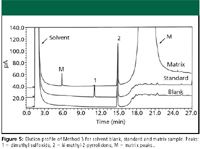
Figure 5
Method 3: Injection of the sample solvent blank and quantitation limit sample showed no interference of N-methyl-2-pyrrolidone with the peak of interest (Figure 5). Improved reproducibility for peak tailing and SPA %RSD were obtained by using N-methyl-2-pyrrolidone as a syringe wash (Table IV). As with Method 2, historically, this method performed adequately in terms of peak area reproducibility; however, SPA %RSD was improved significantly by using N-methyl-2-pyrrolidone as a syringe wash.
After implementing N-methyl-2-pyrrolidone as a syringe wash for these three analytical methods, repeat testing as a result of peak tailing or peak area reproducibility tied to poor syringe performance has been reduced significantly, annually saving over 100 man-hours. In addition, the analyst time required to confirm injections and replace autosampler syringes throughout the analysis of sample sets has been reduced annually by over 80 man-hours.
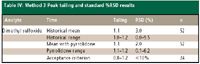
Table IV: Method 3 Peak tailing and standard %RSD results
Conclusions
Using N-methyl-2-pyrrolidone as an additional syringe wash can improve the consistency of GC syringe injections by improving peak symmetry and reproducibility. This, in turn, can lead to a reduction in repeated testing due to failed method acceptance criteria, and a reduction in time needed to confirm consistent injections and replace autosampler syringes during sample analyses.
Acknowledgments
The author wishes to thank the following at Eli Lilly Tippecanoe Labs: Dr. Amy Clough, for her review of and valuable comments on this manuscript; and Jeremy Oelslager, for providing technical support for the experiments and obtaining the chromatograms used in this manuscript.
Bryan E. Bell
Eli Lilly & Company, Tippecanoe Labs, Lafayette, Indiana
Please direct correspondence to Bryan Bell at bbell@lilly.com
References
(1) D.A. Skoog and D.M. West, Fundamentals of Analytical Chemistry, 4th ed. (CBS College Publishing, Philadelphia, 1982), pp. 652, 684.
(2) G.D. Christian and J.E. O'Reilly, Instrumental Analysis, 2nd ed. (Allyn & Bacon, Inc., Boston, 1986), pp. 745, 753–754.
(3) J.V. Hinshaw, LCGC 19(11), 1136–1140 (2001).
(4) J.V. Hinshaw, LCGC 22(3), 252–260 (2004).
(5) L.S. Ettre and J.V. Hinshaw, Basic Relationships of Gas Chromatography (Advanstar Communications, Cleveland, 1993), pp. 3, 115.
(6) J.V. Hinshaw, LCGC, Supplement to: "The Retention Gap Effect," Online Supplement, www.chromatographyonline.com (2004).
(7) J.V. Hinshaw, LCGC 19(10), 1056–1064 (2001).
(8) J.V. Hinshaw, LCGC 22(7), 624–631 (2004).
(9) J.V. Hinshaw, LCGC 24(7), 670–678 (2006).
(10) J.V. Hinshaw, LCGC 19(6), 596–603 (2001).
(11) J.V. Hinshaw, LCGC 24(3), 278–284 (2006).
(12)R. Russell, Pharm. Tech. 28(4), 38–50 (2004).
(13) S.P. Apte and S.O. Ugwu, Pharm. Tech. 27(3), 46–60 (2003).
(14) D.C. Wood, J.M. Miller, and I. Christ, LCGC 22(6), 516–522 (2004).
(15) G.W. Meindersma, Extraction of Aromatics from Naphtha with Ionic Liquids, Thesis, University of Twente, the Netherlands (Febodruk, Enschede, 2005), pp. 2, 75.
(16) M.J. O'Neil (Ed.), The Merck Index, 14th ed. (Merck & Co., Inc., Whitehouse Station, New Jersey, 2006), p. 1054.
(17) D.R. Lide (Ed.), CRC Handbook of Chemistry and Physics, 85th ed. (CRC Press Inc., Boca Raton, 2004), pp. 3-408, 3-409.

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.











